Dear all,
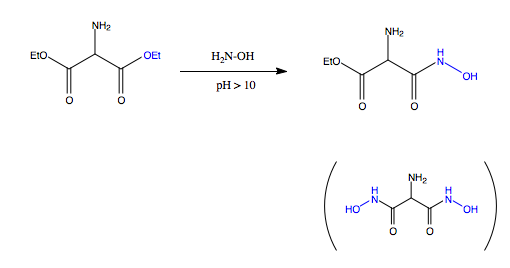
I would like to do the synthetic of hydroxamic acid starting from diester and hydroxylamine. The purpose is to mono-functionalise the -COOEt group and to form the -CO-NH-OH group.
However the problem is that most of the product is di-functionalised compound (even though the less amount of hydroxyl amine has been used).
Do you guys have any suggestion in this case?
Thanks so much!
P.S. The conditions of this kind of reaction are room temperature, stirring, methanol as solvent, and pH > 10.
I would like to do the synthetic of hydroxamic acid starting from diester and hydroxylamine. The purpose is to mono-functionalise the -COOEt group and to form the -CO-NH-OH group.
However the problem is that most of the product is di-functionalised compound (even though the less amount of hydroxyl amine has been used).
Do you guys have any suggestion in this case?
Thanks so much!
P.S. The conditions of this kind of reaction are room temperature, stirring, methanol as solvent, and pH > 10.