Webmanny
Active member
This is really weird.
I have been considering using Kalk in my dosser because I really don't want to mess with my ATO and all the residue that Kalk leaves behind. So, I mixed 1 tsp with a gallon of RODI and started doing a constant dosing of 1ml per hour all day long.
My logic is that doing it this way, it will still use the ATO, but much less and I can adjust the dosing amount as I see fit.
Regardless of the method, the side effect I noticed is that my PH went down yesterday. Please note that nothing else has changed and all parameters are in check. I paid special attention to not change anything else to ensure that I could see the effects of only starting the Kalk and nothing more.
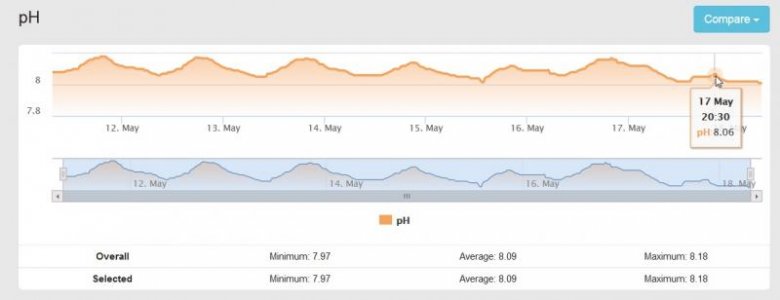
This is the Chart of my PH probe for the past week or so. My PH swings from 8.05 at night to 8.19 during the day, but for some reason, yesterday it only climbed to 8.06 on the high side and so far it has gone down to 8.01 as of right now.
Anyone else has experienced this before with Kalk?

I have been considering using Kalk in my dosser because I really don't want to mess with my ATO and all the residue that Kalk leaves behind. So, I mixed 1 tsp with a gallon of RODI and started doing a constant dosing of 1ml per hour all day long.
My logic is that doing it this way, it will still use the ATO, but much less and I can adjust the dosing amount as I see fit.
Regardless of the method, the side effect I noticed is that my PH went down yesterday. Please note that nothing else has changed and all parameters are in check. I paid special attention to not change anything else to ensure that I could see the effects of only starting the Kalk and nothing more.
This is the Chart of my PH probe for the past week or so. My PH swings from 8.05 at night to 8.19 during the day, but for some reason, yesterday it only climbed to 8.06 on the high side and so far it has gone down to 8.01 as of right now.
Anyone else has experienced this before with Kalk?