awais98
SPS
Im in the process of setting up a new system in the new house.
My current issue is the DI resin gets used up VERY fast.... fast like 4 brute cans of RODI and its done! like 100 gallons only.
Been reading, researching and it seems like CO2 may be a problem.
In theory it seems right. Im in San Antonio and we get out city water supply from underground aquifer waters. Which I belive will be like well water.... very hard and now a lot of co2 apparently.
Researched a lot and seems the best way would be to get the RO water degassed in a storage bin and then pump it through the di resin.
4 questions for the knowledgeable.
1: Just checked my CO2 in RO water ( before the DI resin ) using the ph, alk and it comes out between 5-7 PPM. ( A confounding factor could be, its usually hot here, but this morning it is cool...water may be cooler and CO2 may be higher today? )
Is that high enough for the di resin to be used up? How much DI is used to neutralize X amount of CO2?
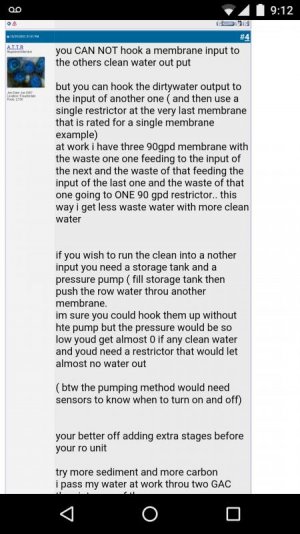
2: If after the RO I get a TDS between 3-5 with this new membrane, can I install ANOTHER RO membrane in SERIES..... so that the first RO membranes product water that is 3-5 TDS is now the input water for the 2nd membrane and so now 99% clearance of 5, will be very near 0, tds will be 0.05....
Will that work INSTEAD of DI resin??? or is the DI resin removing something else that is important. The Co2 if any will be taken care of with the powerhead mixing the salt, or just sitting in the ATO.
It will solve a hell lot of problems.
3: If I decide to regenerate DI resin... and actually seperate cation and anion in different cannisters....does it doecrease efficacy?
I read some where that I could do 3 cans: 1st cation, 2nd anion and 3rd mixed for perfection....
That way atleast the first 2 cannisters will be easy to recharge relatively quickly without removing them from the cannisters.
4: The water storage tanks will be in the garage, where with the cars can get pretty hot... like 100-110 in summer with 2 cars in there....
Will it harm the air stone degassing process....as the air pump will be using the garage air..... basically asking that with the cars not running but just hot in there, does the garage have a lot more CO2?? I know dumb question, but will be important to address in the planning stages...
Thank you for reading!!
Awais
My current issue is the DI resin gets used up VERY fast.... fast like 4 brute cans of RODI and its done! like 100 gallons only.
Been reading, researching and it seems like CO2 may be a problem.
In theory it seems right. Im in San Antonio and we get out city water supply from underground aquifer waters. Which I belive will be like well water.... very hard and now a lot of co2 apparently.
Researched a lot and seems the best way would be to get the RO water degassed in a storage bin and then pump it through the di resin.
4 questions for the knowledgeable.
1: Just checked my CO2 in RO water ( before the DI resin ) using the ph, alk and it comes out between 5-7 PPM. ( A confounding factor could be, its usually hot here, but this morning it is cool...water may be cooler and CO2 may be higher today? )
Is that high enough for the di resin to be used up? How much DI is used to neutralize X amount of CO2?
2: If after the RO I get a TDS between 3-5 with this new membrane, can I install ANOTHER RO membrane in SERIES..... so that the first RO membranes product water that is 3-5 TDS is now the input water for the 2nd membrane and so now 99% clearance of 5, will be very near 0, tds will be 0.05....
Will that work INSTEAD of DI resin??? or is the DI resin removing something else that is important. The Co2 if any will be taken care of with the powerhead mixing the salt, or just sitting in the ATO.
It will solve a hell lot of problems.
3: If I decide to regenerate DI resin... and actually seperate cation and anion in different cannisters....does it doecrease efficacy?
I read some where that I could do 3 cans: 1st cation, 2nd anion and 3rd mixed for perfection....
That way atleast the first 2 cannisters will be easy to recharge relatively quickly without removing them from the cannisters.
4: The water storage tanks will be in the garage, where with the cars can get pretty hot... like 100-110 in summer with 2 cars in there....
Will it harm the air stone degassing process....as the air pump will be using the garage air..... basically asking that with the cars not running but just hot in there, does the garage have a lot more CO2?? I know dumb question, but will be important to address in the planning stages...
Thank you for reading!!
Awais
Last edited: