I recently reexamined some of the literature I have on Marine Ich, paying closer attention to what it says about temperature and its effect on the parasite's known life cycle. As with most studies, precise conclusions can be somewhat ambiguous, but I wanted to share what I have learned and open a discussion regarding this topic.
The first article I reread was the 1997 Colorni and Burgess study, where it states: "œTheront excystment is very asynchronous, occurring between 3 and 72 days." This is the study which the infamous 72 day fallow period is based upon, and it has been suggested it took up to 72 days only because the original experimentation was done in cold water. Indeed, this excerpt from the article seems to support that:
For the reader's reference, 20C=68F and 25C=77F.
However, later in the article (see red highlights below) it states that that the reason for the asynchronous excystment is "unclear". Wouldn't they just say the prolonged excystment (72 days) was due to cooler water temps if they were confident that was the case?
I wouldn't think they'd label it a "œphenomenon" if the simple explanation was that cooler water temps were a contributing factor for asynchronous excystment. It could be because later on they discuss "œcold water intraspecific variants" which only adds to the confusion:
Can a cold water variant infect a reef fish typically found in warmer waters? And vice versa? With regards to temperature, the study was actually more focused on its correlation to trophont/tomont/theront size:
With regards to temperature, the study was actually more focused on its correlation to trophont/tomont/theront size:

The next "œarticle" I reread was a 332 page PDF, written by Peter Burgess in 1992, where he conducted a series of experiments to partially fulfill the requirements for his PhD. Some of the information contained therein is now considered outdated/obsolete, but it still lays the groundwork for most of what we know about the parasite. One such example of possible outdated info is this excerpt:
I know from reading more recent studies that it has been proven ich can go dormant (for up to 6 months, I believe) if temp is lowered, but then become infectious again once the temp is returned to normal. I do not know if the same applies if you were to raise aquarium temperature above 30C/86F. According to "œTable 2" from this source, it would take 40C/104F for 1 hour to disinfect SW ich from your aquarium: https://edis.ifas.ufl.edu/fa164. However, I would think 104F would eradicate even nitrifying bacteria thus "œuncycling" your tank. I don't believe most fish/corals/inverts could handle > 86F (they would ALL need to be removed beforehand), but I do believe bacteria could survive that. The question is how long would you need to keep the aquarium > 86F to eradicate ich from it?
Next up is this table which outlines the development of C.irritans trophonts at different temperatures:

However, I found nothing above to be useful for our purposes since most of the animals we keep couldn't survive in 17C/62.6F water.
Finally, the study had this to say regarding temperature and immune response to SW ich:
Two other articles I have not read on the subject, so I will just copy & paste their abstracts below:
Studies on cryptocaryoniasis in marine fish: effect of temperature and salinity on the reproductive cycle of Cryptocaryon irritans - Journal of Fish Diseases Volume 2, Issue 2, pages 93"“97, March 1979
Influence of Temperature and Host Species on the Development of Cryptocaryon irritans
B. K. Diggles and R. J. G. Lester
The Journal of Parasitology
Vol. 82, No. 1 (Feb., 1996), pp. 45-51
Conclusions: Some of the information above is unusable for our purposes, as many of the animals we keep will not live in the experimental temperatures shown to have a negative impact on ich's life cycle. Although, those with cold water SW tanks battling ich might find it useful. The main thing I was looking for was whether or not the recommended 72 day fallow period is greatly exaggerated due to experimentation being conducted at cooler water temps. And while I admit there is some evidence to support it is, I also believe there are other variables in play which determines how long it can take for all the theronts to be released (or rupture) from their respective tomonts. I also cannot discount the numerous anecdotal accounts of 72 day fallow failures, or chalk every single one of them up to cross contamination or some other mental error on the part of the hobbyist. In short, we probably don't know as much about ich as we think we do.
I do think, however, that it would be prudent to monitor aquarium temp while going fallow. You probably want it to be at least 77F, and it is possible that running it at 80-82F will speed up ich's life cycle and increase your chances of having a successful fallow period. At the very least, it does no harm as most corals/inverts handle 80-82F just fine (except for possibly certain SPS.)
Whether you decide to go fallow for the entire 72 days (actually 76 if you factor in more than just the tomont stage), or roll the dice on a shorter duration is entirely up to you. For those who opt for the latter, Table 1 (below) provides some useful info taken from here: http://atj.net.au/marineaquaria/marineich.html
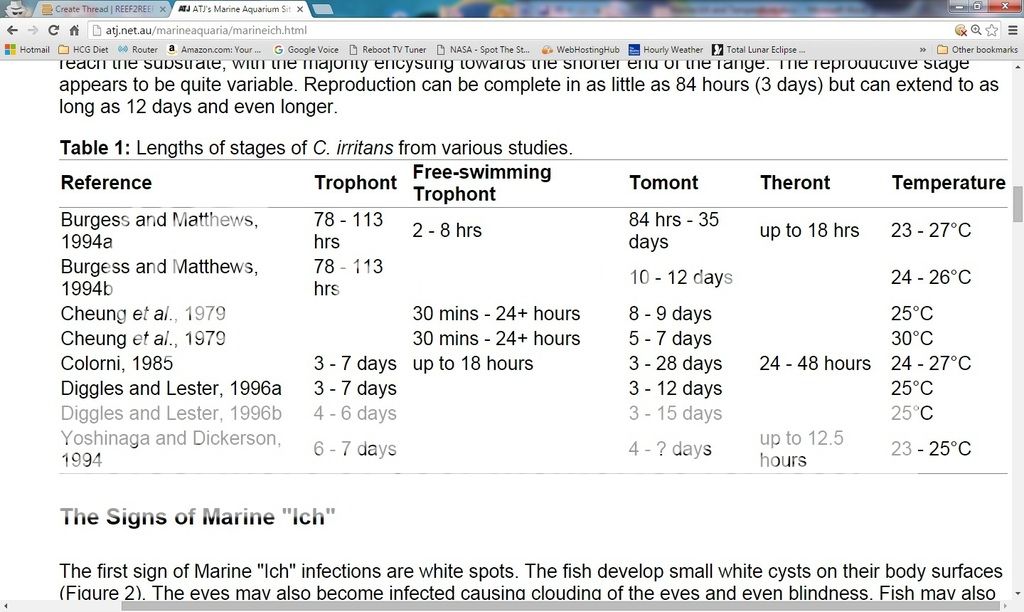
It shows lengths of stages of C. irritans from various studies before the 1997 Colorni and Burgess Study. In these listed studies, 35 days was the longest time it took for theront release (Burgess and Matthews, 1994a). So, 45 days fallow should be sufficient for most garden variety strains of ich so long as temp is 77F or greater during the entire fallow period.
One last thing I wanted to mention is something I said previously - about ich going dormant in lower temps and then becoming infectious again once the temp is returned to normal. That information is quoted below and was extracted from here: https://edis.ifas.ufl.edu/fa164
So let's now begin a lively discussion, debate, more info presented, etc. on Marine Ich and Temperature!
The first article I reread was the 1997 Colorni and Burgess study, where it states: "œTheront excystment is very asynchronous, occurring between 3 and 72 days." This is the study which the infamous 72 day fallow period is based upon, and it has been suggested it took up to 72 days only because the original experimentation was done in cold water. Indeed, this excerpt from the article seems to support that:
The Australian trophonts stayed on the fish longer, tomonts took longer to excyst and the theronts were larger when fish were infected at 20C compared to 25C (Diggles and Lester, 1996a).
For the reader's reference, 20C=68F and 25C=77F.
However, later in the article (see red highlights below) it states that that the reason for the asynchronous excystment is "unclear". Wouldn't they just say the prolonged excystment (72 days) was due to cooler water temps if they were confident that was the case?
Even under identical incubation conditions tomonts vary considerably in the time required to form theronts (Nigrelli and Ruggieri, 1966; Colorni, 1992; Burgess and Matthews, 1994a; Diggles and Lester, 1996b). Thus, theront excystment is very asynchronous, occurring between 3 and 72 days and peaking at 6 ± 2 days (Colorni, 1992). This differs significantly from I. multifiliis, where the theront excystment takes only 18-24 h at 23C (Dickerson and Dawe, 1995).
The reason for asynchronous excystment is unclear. There is no relationship between the tomont size and excystment time (Nigrelli and Ruggieri, 1966; Colorni, 1992; Diggles and Lester, 1996a,b). In fact, a large and a small tomont may produce theronts at the same time, even though the smaller tomont undergoes fewer divisions. When tomites do not form until at least 2 weeks, a mass of endoplasm remains undifferentiated and fewer live theronts are produced (Colorni, 1992). Whatever the cause, asynchronous excystment prevents simultaneous exhaustion of all tomonts, facilitates theront dispersal in time and appears so advantageous to C. irritans that the phenomenon should be interpreted as a strategy for survival (Colorni, 1985).
I wouldn't think they'd label it a "œphenomenon" if the simple explanation was that cooler water temps were a contributing factor for asynchronous excystment. It could be because later on they discuss "œcold water intraspecific variants" which only adds to the confusion:
Cryptocaryon irritans was considered to be restricted to warmwater marine environments. However Diamant et al. (1991) found that C. irritans has a counterpart in the cooler waters of the eastern Mediterranean and Diggles and Lester (1996c) collected Cryptocaryon-infected fishes from Moreton Bay, Queensland, Australia, where the water temperature can fall to 15C. By comparing the rDNA sequences of isolates from Australia, Israel and the USA, Diggles and Adlard (1997) confirmed the existence of warm water and cold water intraspecific variants of C. irritans.
Can a cold water variant infect a reef fish typically found in warmer waters? And vice versa?

The next "œarticle" I reread was a 332 page PDF, written by Peter Burgess in 1992, where he conducted a series of experiments to partially fulfill the requirements for his PhD. Some of the information contained therein is now considered outdated/obsolete, but it still lays the groundwork for most of what we know about the parasite. One such example of possible outdated info is this excerpt:
The distribution of C.irritans in the wild appears to be limited by temperature. Under aquarium conditions, C.irritans has not been shown to develop or transmit below 19C to 20C (Wilkie and Gordin, 1969; Cheung et al., 1979) or above 30C (Cheung et al., 1979). Based on this information, it is considered that C.irritans is restricted to warmwater marine environments, although recent observations by Diamant et al. (1991) suggest that C.irritans may have a counterpart existing in the cooler waters of the eastern Mediterranean. This assumption was based on reports of disease outbreaks caused by a Cryptocaryon- like ciliate which was believed to have originated from cultured fish stocks from Cyprus and northern Israel.
I know from reading more recent studies that it has been proven ich can go dormant (for up to 6 months, I believe) if temp is lowered, but then become infectious again once the temp is returned to normal. I do not know if the same applies if you were to raise aquarium temperature above 30C/86F. According to "œTable 2" from this source, it would take 40C/104F for 1 hour to disinfect SW ich from your aquarium: https://edis.ifas.ufl.edu/fa164. However, I would think 104F would eradicate even nitrifying bacteria thus "œuncycling" your tank. I don't believe most fish/corals/inverts could handle > 86F (they would ALL need to be removed beforehand), but I do believe bacteria could survive that. The question is how long would you need to keep the aquarium > 86F to eradicate ich from it?
Next up is this table which outlines the development of C.irritans trophonts at different temperatures:

However, I found nothing above to be useful for our purposes since most of the animals we keep couldn't survive in 17C/62.6F water.
Finally, the study had this to say regarding temperature and immune response to SW ich:
The kinetics of the antibody response to C.irritans is likely to be influenced by temperature, within the physiological limits of the host, as is well recognised for teleosts antibody responses in general (Rijkers et al., 1981; Rijkers, 1982; Bly and Clem, 1992). The timing may also vary according to the host species, and there is some evidence to support this (Rijkers, 1982). Sailendri and Muthukkaruppan (1975), using Tilapia mossambica, have shown that under tropical conditions (30°C) a primary antibody response can be elicited within as short a period as five days after exposure to antigen. Although the species of mullet used here has a southerly distribution, extending to the Mediterranean (Lythgoe and Lythgoe, 1971), the speed of its immune response might not be representative of tropical marine fish species normally encountered by C.irritans. The delay in antibody response, recorded here, following intraperitoneal injection could also be attributed to temperature, as mullet immunized by this route were maintained at 5-10°C lower than those exposed to C.irritans by natural infection.
Two other articles I have not read on the subject, so I will just copy & paste their abstracts below:
Studies on cryptocaryoniasis in marine fish: effect of temperature and salinity on the reproductive cycle of Cryptocaryon irritans - Journal of Fish Diseases Volume 2, Issue 2, pages 93"“97, March 1979
Abstract. Trophonts of Cryptocaryon irritans Brown from infected three-spot damselfish, Dascyllus trimaculatus Ruppell, were kept at temperatures ranging from 7 to 37°C to observe encystment and development of the tomites. At 30, 25 and 20°C, the percentage of trophonts that had encysted in 16 h were 70, 77 and 64% respectively; at 37°C, 44% encysted and at 7°C only 10% had encysted.
The optimum temperature for excystment was 30°C; 50% excysted in 5 days and 100% in 7 days. At 25°C, 60% of the tomites started to excyst on the eighth day, and 70% on the ninth day. At 20°C, 10% started to excyst on the ninth day, reaching 40% on the tenth day. No excystment occurred at 37 and 7°C.
Newly encysted tomonts were placed in various dilutions of sea water (31 %0) and kept at temperatures ranging from 7 to 37°C. Low salinities, i.e. 16%0 and lower caused tomonts to rupture. At 37, 20 and 7°C, 35% of the tomonts started to rupture immediately in 50% sea water, while at 30 and 25 C, 30% of the tomonts ruptured in 25% seawater. However, none of the cysts developed normally at these dilutions. The percentage rupturing increased with decreasing salinity.
Influence of Temperature and Host Species on the Development of Cryptocaryon irritans
B. K. Diggles and R. J. G. Lester
The Journal of Parasitology
Vol. 82, No. 1 (Feb., 1996), pp. 45-51
Abstract. The course of infection of the parasitic ciliate Cryptocaryon irritans was followed on Lates calcarifer and Macquaria novemaculeata at 20 and 25 C. The parasite was originally isolated from locally caught Acanthopagrus australis. At 20 C trophonts stayed on the fish longer, tomonts took longer to excyst, and the resulting theronts were larger than at 25 C. On L. calcarifer at 20 C, trophonts grew slowly at first but eventually became significantly larger (mean tomont diameter 466 x 400 µm) than at 25 C (mean diameter 373 x 320 µm). On M. novemaculeata, trophonts never grew as large as on L. calcarifer and at 20 C they grew poorly. The number of theronts produced per tomont was directly related to the size of the tomont but was not influenced by incubation temperature. The tomont incubation period was not related to the diameter of the tomont but was significantly influenced by the host origin of the tomont. Theront size was also significantly affected by the host origin of the tomont but not the diameter of the tomont. These results show that C. irritans exhibits variability in morphometrics on different hosts and under different temperature conditions. This variability needs to be taken into account if utilizing morphometric data for separating strains of C. irritans.
Conclusions: Some of the information above is unusable for our purposes, as many of the animals we keep will not live in the experimental temperatures shown to have a negative impact on ich's life cycle. Although, those with cold water SW tanks battling ich might find it useful. The main thing I was looking for was whether or not the recommended 72 day fallow period is greatly exaggerated due to experimentation being conducted at cooler water temps. And while I admit there is some evidence to support it is, I also believe there are other variables in play which determines how long it can take for all the theronts to be released (or rupture) from their respective tomonts. I also cannot discount the numerous anecdotal accounts of 72 day fallow failures, or chalk every single one of them up to cross contamination or some other mental error on the part of the hobbyist. In short, we probably don't know as much about ich as we think we do.
I do think, however, that it would be prudent to monitor aquarium temp while going fallow. You probably want it to be at least 77F, and it is possible that running it at 80-82F will speed up ich's life cycle and increase your chances of having a successful fallow period. At the very least, it does no harm as most corals/inverts handle 80-82F just fine (except for possibly certain SPS.)
Whether you decide to go fallow for the entire 72 days (actually 76 if you factor in more than just the tomont stage), or roll the dice on a shorter duration is entirely up to you. For those who opt for the latter, Table 1 (below) provides some useful info taken from here: http://atj.net.au/marineaquaria/marineich.html

It shows lengths of stages of C. irritans from various studies before the 1997 Colorni and Burgess Study. In these listed studies, 35 days was the longest time it took for theront release (Burgess and Matthews, 1994a). So, 45 days fallow should be sufficient for most garden variety strains of ich so long as temp is 77F or greater during the entire fallow period.
One last thing I wanted to mention is something I said previously - about ich going dormant in lower temps and then becoming infectious again once the temp is returned to normal. That information is quoted below and was extracted from here: https://edis.ifas.ufl.edu/fa164
Temperatures for optimal growth of most strains of Cryptocaryon appear to be about 23"“30°C (73.4"“86°F) (Dickerson 2006; Yoshinaga 2001), although active infections at 15°C (59°F) have been documented (Diggles and Lester 1996). Encysted stages, off the host (tomonts), were also observed to survive for 2"“4 weeks under experimental hypoxic conditions (24% oxygen saturation); these released free-swimming infective stages (theronts) 10"“11 days after excystment (Yoshinaga 2001).
A more recent study demonstrated that two life stages of one strain of Cryptocaryon (trophonts, i.e., the feeding stage during which the parasite can be found on the fish, and tomonts) survived dormant for 4"“5 months at 12°C (53.6°F), and, after the water temperature increased to 27°C (80.6°F), developed and infected fish (Dan et al. 2009).
So let's now begin a lively discussion, debate, more info presented, etc. on Marine Ich and Temperature!
