bulldogin04
New member
Ok Here's the deal. I recently got back into the hobby and setup my old 30 gallon cube. I got new lights (LED of course, bye bye old compacts), new reef octopus skimmer and went with my old trickle filter sump. Ran the tank through its break-in cycle over year with a small bio load, pair of clowns and a cleaner wrasse, plus artificial "purple" live rock (not recommended BTW).
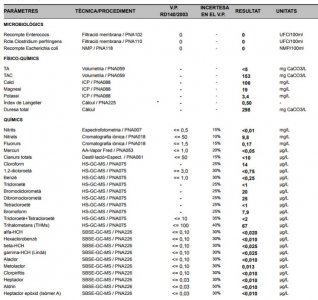
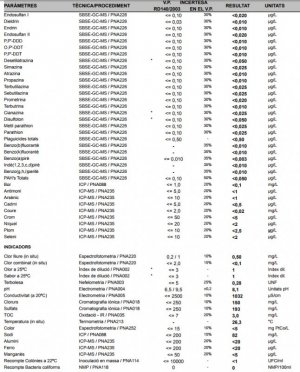
After the first liberal break-in year, I started to notice a white slime algae and treated it like any other diatom break out; lights out for 2 days reduced the already conservative feeding. Yes, it would get knocked back but always managed to come back after a week or so. So I did what every other reef keeper does - Check your water parameters. I'm not a water quality nazi, but I don't neglect my tank mates cause that's cruel; but every time I checked things the only abnormal parameter was pH at 7.8. Ok, 7.8 is low, but not something to jump up and down about, time to go with some kalwasser. So here's where things get interesting.
From the start, I used distilled water by the gallon as my water source (yes, I recycled the plastic) and I read somewhere that the plastic in those jugs leaches out a certain chemical that cyano-bacteria like (totally bogus). I was starting to get paranoid at this point with such a persistent problem so I figured its time to go RO. When I switched to a 6 stage RO/DI water kit, I noticed that my pH was now dipping below 7.8 on occasion. That is something to worry about.
Now we go off the deep end in this story. I, unlike most people, have access to lab grade pH and DO meters (calibrated daily) and know the folks that work in the water utility. One day I bring in a grab sample from my tank and my tap and my RO water. Tank 7.8, tap water 6.8 and fresh RO water 6.2, YES 6.2! So I give the operations manager a call and set up a meeting and try to understand what is going on here.
Put on your thinking cap cause this is were the chemistry comes in. According to the Water Treatment Operations Manager, the water they put into their distribution system is about 7.5. The source water is a surface lake and not ground water. The source water coming in slightly acid and ranges from 6-6.8 but can vary a lot with precipitation events. The treatment process used here is chlorine disinfection, followed by ozone, then UV sterilization then chloramines (filtration? forget it!), followed by another dose of free chlorine for residual.
Q: So how do you go from 6.5 source water to 7.5 in distribution? A: Well we add sodium potash (KOH) to buffer and raise pH as well as adding phosphoric acid (H3PO4) for corrosion control in the water mains. Now this is making sense, the RO unit is removing all these items from the tap water hence the drop in the tap water pH as it exits the RO unit. However, the free hydrogen atom remains in solution from the phosphoric acid and will readily bind with atmospheric CO2 and forming carbonic acid (H2CO3). This keeps my pure RO water pH below 7. Normal RO water is going to go acid anyway, mine is just extra hungry for CO2.
Remember how I said the source water is from the surface, not ground water? The surface water is very pure, that's good right? Nope, it has absolutely zero buffering capacity; hence the large fluctuations in pH through out the water treatment process and in the end, the acid water coming out of my RO unit for saltwater mixing. Most salt mixes are designed to be mixed with water with a pH of 7 or greater.
So here's how I got my white slime algae / diatom problem under control. I have to continually add kalwasser at about 2 times the normal rate to maintain higher acceptable pH. The higher pH ranges have reduced the slime algae problem significantly. The added bonus is my leather coral is actually getting bigger, xenia are splitting and growing, calcareous algae are starting to grow. So after almost 2 years I finally have my tank water chemistry working right.
So the moral of the story is know your source water and water chemistry!
Thanks for reading
After the first liberal break-in year, I started to notice a white slime algae and treated it like any other diatom break out; lights out for 2 days reduced the already conservative feeding. Yes, it would get knocked back but always managed to come back after a week or so. So I did what every other reef keeper does - Check your water parameters. I'm not a water quality nazi, but I don't neglect my tank mates cause that's cruel; but every time I checked things the only abnormal parameter was pH at 7.8. Ok, 7.8 is low, but not something to jump up and down about, time to go with some kalwasser. So here's where things get interesting.
From the start, I used distilled water by the gallon as my water source (yes, I recycled the plastic) and I read somewhere that the plastic in those jugs leaches out a certain chemical that cyano-bacteria like (totally bogus). I was starting to get paranoid at this point with such a persistent problem so I figured its time to go RO. When I switched to a 6 stage RO/DI water kit, I noticed that my pH was now dipping below 7.8 on occasion. That is something to worry about.
Now we go off the deep end in this story. I, unlike most people, have access to lab grade pH and DO meters (calibrated daily) and know the folks that work in the water utility. One day I bring in a grab sample from my tank and my tap and my RO water. Tank 7.8, tap water 6.8 and fresh RO water 6.2, YES 6.2! So I give the operations manager a call and set up a meeting and try to understand what is going on here.
Put on your thinking cap cause this is were the chemistry comes in. According to the Water Treatment Operations Manager, the water they put into their distribution system is about 7.5. The source water is a surface lake and not ground water. The source water coming in slightly acid and ranges from 6-6.8 but can vary a lot with precipitation events. The treatment process used here is chlorine disinfection, followed by ozone, then UV sterilization then chloramines (filtration? forget it!), followed by another dose of free chlorine for residual.
Q: So how do you go from 6.5 source water to 7.5 in distribution? A: Well we add sodium potash (KOH) to buffer and raise pH as well as adding phosphoric acid (H3PO4) for corrosion control in the water mains. Now this is making sense, the RO unit is removing all these items from the tap water hence the drop in the tap water pH as it exits the RO unit. However, the free hydrogen atom remains in solution from the phosphoric acid and will readily bind with atmospheric CO2 and forming carbonic acid (H2CO3). This keeps my pure RO water pH below 7. Normal RO water is going to go acid anyway, mine is just extra hungry for CO2.
Remember how I said the source water is from the surface, not ground water? The surface water is very pure, that's good right? Nope, it has absolutely zero buffering capacity; hence the large fluctuations in pH through out the water treatment process and in the end, the acid water coming out of my RO unit for saltwater mixing. Most salt mixes are designed to be mixed with water with a pH of 7 or greater.
So here's how I got my white slime algae / diatom problem under control. I have to continually add kalwasser at about 2 times the normal rate to maintain higher acceptable pH. The higher pH ranges have reduced the slime algae problem significantly. The added bonus is my leather coral is actually getting bigger, xenia are splitting and growing, calcareous algae are starting to grow. So after almost 2 years I finally have my tank water chemistry working right.
So the moral of the story is know your source water and water chemistry!
Thanks for reading