juliovideo
Premium Member
THE POINT ON THE AUTOTROPHIC DENITRATATION ON SULPHUR.
By Marc LANGOUET ing ENSCR
In confined surroundings, like that consisted an aquarium, the regular contribution of foods which necessarily contain a quantity, more or less important, nitrogenized matters will lead, ineluctably, early or late with a content nitrates incompatible with the life of the lodged species.
This phenomenon is now well-known of the majority of the aquarists, in particular those which maintain the récifaux vats, the tolerance of the corals to nitrates being particularly weak compared to other living organisms.
Various solutions were proposed to mitigate this problem: water change, filter algae.... Most common of all is probably the scummer, bases Berliner method which consists in eliminating the maximum of nitrogenized matters before they are not transformed into nitrates.
Nevertheless these methods are not without difficulties and always do not allow to regulate the problem easily. I think particularly of the vats rather or very charged out of fish or those which lodge corals or invertebrates requiring of the frequent contributions of food (considerable of gorgones or very beautiful corals such as Tubastrea aurea, the family of Dendronephtya or Carotalcyon sagamianum).
These splendid animals are very seldom high out of aquarium because owing to the fact that it is advisable to nourish them very regularly (what can be automated) and that that led very quickly tohigh nitrate rates.
I will explain here the two original methods which I developed and of which the use could be spread quickly.
1° the autotrophic denitratation on sulphur
A article published on the site MARCH the 18/5/98 and written by Christophe SOLER summarizes the method rather well. I will thus be satisfied simply to specify the history and to add some information. It is my former professor Guy Martin of the Higher National School of Rennes which is at the origin of this idea; it applied it to the soft water treatment intended for the network of adduction.
From the 1991 I tested this sea water method what was new especially that we did not know if it would have consequences in terms of toxicity with respect to the species present.
It is only fine 94 which, extremely 3 years of experiments without apparent toxicity on many vats and species present at my residence, I proposed this Michel Hignette method preserving of the aquarium of the MAAO. A pilot was launched there by my care.
Since then, the experimentation was carried out on a large scale, as well with the MAAO as with the Large Aquarium of Saint-Malo from which I ensured the technical and scientific direction on June first, 1996 mid-December 1997.
I make a point of specifying that I use since 1996 of sulphur in ball of average diameter 3,5 millimetres marketed by SESOLàSt Herblain(44). This presentation is much easier to use than to start from sulphur out of bar and to crush it with the hammer.
The quantity of sulphur to be used is a function of the initial nitrate rate at the time of the startup and the quantity of food brought. I consider that a volume of sulphur equal to 1% of the total volume of water to be treated is sufficient when the initial nitrate rate is lower than 50 mg/l (NO3 -).
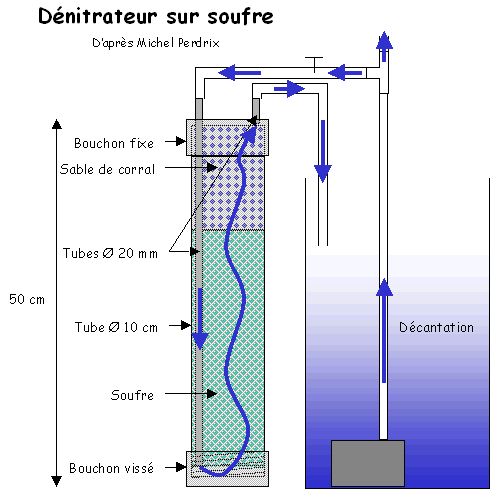
The water flow which can cross the sulphur column depends on the nitrate rate of water to treat: more there is nitrate plus the flow in entry must be weak or else you will find a part of nitrates at exit of column.
You can count for starting on an order of magnitude of 1 L per hour and liter of sulphur in the column. You will adjust then in the following way:
- For a too low flow you will have an egg odor rotted at exit of column due to a production of sulfurous hydrogen (H2S) what will seldom arrive and for very low flows really.
- For a too strong flow you will detect nitrites or nitrates in the water of exit whereas regulated well you can obtain 0 mg/l nitrate at exit.
However the experiment shows that the system is very tolerant as for the output control, flow which will be able to go up to five liters hour per liter of sulphur.
- the water sent on the column can come from a derivation of the aerobic filter or directly of the vat. The column must allow the exhaust of produced nitrogen: for this reason a vertical water circulation upwards appears preferable to me with output control in entry of column and not at exit. The exit of column can be opened with the free air. Water leaving the very acid column of sulphur perhaps but according to my experiments a degasification of this water (for example by injection of air using a diffuser) makes it possible to find a pH close to that of entry. The acidity of this water is thus at least mainly related to the presence of carbon dioxide; from where the idea to use this water to produce an engine with limestone by making it pass in one second (and even a third) column identical in the face to that containing sulphur but this time filled of maërl or coral sand of low granulometry (same sand as that which you use to furnish the bottom with your vat).
This water also contains sulphates in quantity slightly higher than the rate in entry but it was never observed of consequence in 7 years of experimentation even on vats not undergoing any water change during years. It is necessary to say on this subject which an error slipped into the publication made jointly with the MAAO: the sulphur rate present in natural sea water is of almost 900mg/l; this sulphur is present in the form of sulphate (SO4- -)soit approximately 2,65 sulphate g/l, quantity which with it only can explain why the contribution of the sulphate system will be without notable consequence.
2° the Jaubert method doped with sulphur.
The Jaubert method is, now, better and better known; and I use it since 1994. It consists in carrying out the reduction of nitrogen nitrates and the engine with limestone directly in the aquarium. The reduction occurs in the low part of a thick layer of sand from 8 to 10 cm (according to personal communication of J Jaubert). This method functions very well; nevertheless, it seems limited to the fish aquariums not very charged or rather little nourished, probably for lack of matter organic in the low part of sand factor limiting the development of the anaerobic bacteria which transform nitrates nitrogenizes some by nourishing carbonaceous compounds (organic matter).
It also seems that only the quite enlightened aquariums can function according to this method without one having today indisputable explanation on this subject.
Lastly, it is necessary that the surface of the ground of the aquarium which is covered by the decoration does not exceed approximately 25 % (always according to the information communicated by J.Jaubert).
If, for a reason or another, of nitrates are present in a persistent way in water, one can always dope the system to make them disappear, more quickly, by accelerating the bacterial process by organic matter introduction into the low layer of sand (glucose for example introduced by a cane penetrating under the layer and emerging above the surface of the water of the aquarium).
To make function this Jaubert method beyond its limits i.e. in a very nourished and little or not lit aquarium, without having to add glucose under the layer, while preserving its advantages to know extreme simplicity, not from external engine, not from flow to be regulated, engine with limestone to incorporate, etc the idea came to me, a few years ago, to free me from the limiting factor which the quantity of organic matter constitutes present in the low part of sand by doping the system with a small layer of sulphur in balls placed at this level. This makes it possible to make function the system in an autotrophic carbon way and either only heterotrophic.
This device that I tried out successfully consists of a traditional basic grid style filters under sand but without suction pipe (water crosses the ballasting only by natural diffusion between the sand grains as in the Jaubert method).
The experimental protocol that I set up at the end of August 1998 was as follows:
I have completely remakes an aquarium of 100 liters which had turned for several years and which had 6 to 8 cm of coral sand and the alive rocks. It should be noted that this vat, although installed according to principles' of the Jaubert method, saw its rate nitrate to go up for the periods of nourrissage.
Of the same vacuum of any animal and not nourished during months its rate of nitrate did not drop. It is the perfect of a vat Jaubert little or not lit example and which does not function sufficiently quickly, undoubtedly for lack of nutrients for the anaerobic bacteria present in the layer.
I went up the whole while intercalating between the basic grid and the ballasting a layer of sulphur in balls of approximately 0,5 cm thickness placed between two layers of plastic mosquito net netting.
The 65 to 70 liters of sea water of the vat had 35 mg/l nitrate(NO3 -). I employed again this water to fill the vat. Five days later, the vat showed a nitrite rate of a value higher than 10 mg/l. Sand used being alive sand, already sown in aerobic bacteria, it is the first stage of the anaerobic transformation of nitrite nitrates which was occurring on sulphur and not the transformation of ammonia into nitrite like traditionally in a vat lately installed.
The tenth day, the nitrite rate was about 1 Mg per liter; the transformation of nitrites into gas nitrogen was thus in hand. But in a system of this type, it is logical that the water circulation occurring by natural diffusion through the ballasting the evolutions measured in the water of the vat are slower than those noted on the outlet side of an engine with sulphur where one forces water to cross the filter.
The eleventh day, there did not remain any more nitrite and the rate of nitrate had fallen to 5 mg/l.
The twelfth day, the nitrates were not detectable any more whole (lower than 1mg/l).
With the eightieth day the vat is always free from nitrate although it receives each day a cube of artémias frozen of approximately 1 Cm3 what is high a enough contribution for 65 liters of water. The tubastréa auréa present in this vat for 2 months has been practically open all the day appreciating the fort running which reigns in the vat and the daily contribution of food. It is the same for the actinias équina and the two specimens of carotalcyon sagamianum. The pH is about 7,7 because of the presence of carbon dioxide (a degasification makes go up the pH) but that does not seem to obstruct the invertebrates. KH lies between 6 and 7. It is also that of the water of the English Channel which was used at the origin to fill this vat.
This method, very interesting, could perfectly installation in the additional decantation of an existing vat of the kind that one is not obliged to remake the whole of the vat. The relatively low pH surely will reject some among you, but it should be known that with the Jaubert method, the pH and KH are in general definitely low than with the Berliner method (at least the morning for the pH). It is without notable consequence on the animals. My vat is not enlightened (if it is not by the light of the part) it is not ventilated differently than by a normal exchange with the interface air-water, this interface being brewed little besides since I privileged the current on the corals in order to bring their food to them.
In an enlightened and brewed aquarium surfaces some or having diffuser, carbon dioxide dissolves will be eliminated better and the pH will be surely higher the more so as it is undoubtedly not necessary to have sulphur on all the surface of the vat such as I did it.
I am persuaded for my part that this presence of CO2 in quantity in water is not inevitably a problem but the future will say it to us. As soon as I can acquire some I add in this aquarium some Dendronephtyas, ombrophilous gorgones, Sphaerella krempfis, sponges etc.... the food as for it must be able to be automated and thus more regular using slot-machine bus of simple flakes for fish are enough to make open Tubastréa auréa.
Thank you to make me share of your experiments in this direction.
To follow.
Marc LANGOUET.
6/11/98
NB 1
Autotrophic:qui does not require the contribution of an energy or nutrients external with the system.
Heterotrophic: who requires it
NB 2 You can make share of your comments or of the results of your experiments is directly with
Marc LANGOUET
Guimorais
35350 St Coulomb
tél.+fax. 02 99 89 41 69
Maybe with the regional contact of Reef France in Brittany which will transmit
Pierre ZMIRO
17 street of the station
49440 Candé
Tel.. 02 41 94 92 10
Fax 02 41 94 92 11
FBZ@wanadoo.fr E-mail
-------------------------------------------------------------
Various questions, Answers & Infos
Q : Degasification (exhaust of produced nitrogen) is evoked by Marc in his article. Not having decantation in my vat, I want to place the column in bottom in the piece of furniture; it will be thus completely full of water. I add that I will fill the column of sulphur and Maërl with 50/50%. I intend to reject water to treat with the free air with the top of the water level of the vat, but will that be enough for degasification? Can I function thus without risk while being also effective?
R of Marc: There is no problem so that the column is full of water; It is necessary that circulation is done upwards in the column sulphur /maërl, but it should be noted that it because the maërl will be reduced out of mashed potaties with time and will have to be thrown the whole is not a very good idea because that will be clogged... Better is worth to separate sulphur and sand because sulphur can be preserved: it does not worsen.
Q : Should sulphur be added regularly?
R of Marc: There is a sulphur consumption but it is very weak it corresponds to the transformation:
4NO3 + 3S = 2 N2 + 3 SO4
(that is more complex but the assessment returns to that)
Actually I did not add sulphur during years nor even less changed sulphur.
Q : With what is used exactly the air intake and it is fundamental?
R of Marc: The air intake is not there that to facilitate the exhaust of nitrogen. If one works on nitrate rates enough élevés.Mais if the nitrate rate in entry of the engine is weak this nitrogen degasification will be weak and I think that it will not pose problem to send this water in an Visio-engine but the experiment remains to be made.
Q : Which is the cost of Sulphur?
R of Marc: The cost of sulphur is about 10 to 30 FF/kg
Q : I made an engine according to articles' of MARCH with 2 columns of 5 liters; a container of the sulphur balls, and the other containing a calcareous substrate standard engine with limestone gross granulometry. In eight day, the nitrates which were approximately 25 mg/l in my vat of 400 liters, were found reduced to Zero at exit of column. Since, approximately one month after its startup, the nitrate rate ranges between 0 and 5 mg/l in the vat. It is really effective!!
But as anything is not easy in récifal, it exists other parameters quite as important, which can be, them also, touched by the engine with sulphur. For calcium first of all, I had 410 mg/l approximately at the beginning, and all
continuously as usual my contribution by lime water for evaporation (5 liters per week approximately because my closed vat evaporates little, recall: lighting exclusively in fluos). I have at the end of one month 490 mg/l at exit of column!! It, do I have is brilliant to suppose that it is the limestone column which makes effect?
On the other hand, the pH, is to him since the beginning lower by 0,6 to 0,8 at exit of column compared to the vat. Thus, it is descended in the vat from 8,2 to 7,8 at the end of one month (7,05 at exit of column). What can I make to increase the pH without upsetting properly the animals? This fall of pH arrètera-you it one day? This problem is it inherent with the systême of denitratation on sulphur and thus that to make to stabilize the pH in the surrounding of 8,3/8,4?
R of MARCH: The flow of the engine is not indicated. Perhaps can one play above (with the rise or the fall) in order to reduce the amount of acid distributed in the aquarium, to test by measuring the pH of exit, as for an engine with limestone. Another possibility: Always as in an engine with limestone, Si the sand of coral is too large, water passes too quickly inside, with a heat-transferring surface miniumum, which does not make it possible to dissolve sand enough and thus to reduce the acidity of water. For remèdier with that, that is to say to increase the length of the circuit in the coral sand, or more simply to take sand of granulomètrie between 1 and 5 mm.
Q : How gèrer production of nitrites at the time of the launching of the engine?
R of Marc: One should not especially work has flow raised at the beginning during the launching phase because the reaction is anaerobic (without oxygen), and thus if you work with high flow there will not be average to pass in anaerobiose and the reaction will not start. On the contrary it is necessary to reduce the flow to lowest possible (of the order of a drop for the second for 1 sulphur L, and thus the produced nitrites can be sent in the vat without risk: sight their small quantity they will be transformed by the aerobic filtration of the vat into nitrates. At the end of 3 to 5 days the nitrites will disappear from water at exit and you will be able to increase the flow until reaching gradually the cruising speed while taking care during this phase of growth not to make reappear the nitrites by a flow too fast Les nitrates will fall them have zero as soon as the nitrites disappear and have condition of not too quickly increasing too much nor the flow; There is no water change to make. The quantity of nitrites at the time of starting is related to the quantity of nitrates in the water of the vat: if the nitrates are weak, the nitrites will be it too.
Q : Is it necessary to take special precautions when one stops an engine with Soufre?
R of C Soler: If you stop the denitrator by leaving this one filled, it is necessary to let run several times its volume for the re-starting (hydrogen sulphide), you will see that really been able. When the water which leaves the engine does not feel any more you can let it run again in the vat.
--------------------------------------------------------------------------------
Warning of MARCH :
1 the use of this type of system, after one year of experiment in many aquarists, seems always promising, but generates some perverse effects that it is necessary for us to learn with maitriser:
- KH drops
- calcium surdose important (800 mg/l by ex, but any excess is harmful)
- various difficulties
If the fall of the nitrate rate is obvious, remains some details to be developed. MARCH hopes to be in measurement to publish soon the synthesis of various experiments on the subject
-----------
By Marc LANGOUET ing ENSCR
In confined surroundings, like that consisted an aquarium, the regular contribution of foods which necessarily contain a quantity, more or less important, nitrogenized matters will lead, ineluctably, early or late with a content nitrates incompatible with the life of the lodged species.
This phenomenon is now well-known of the majority of the aquarists, in particular those which maintain the récifaux vats, the tolerance of the corals to nitrates being particularly weak compared to other living organisms.
Various solutions were proposed to mitigate this problem: water change, filter algae.... Most common of all is probably the scummer, bases Berliner method which consists in eliminating the maximum of nitrogenized matters before they are not transformed into nitrates.
Nevertheless these methods are not without difficulties and always do not allow to regulate the problem easily. I think particularly of the vats rather or very charged out of fish or those which lodge corals or invertebrates requiring of the frequent contributions of food (considerable of gorgones or very beautiful corals such as Tubastrea aurea, the family of Dendronephtya or Carotalcyon sagamianum).
These splendid animals are very seldom high out of aquarium because owing to the fact that it is advisable to nourish them very regularly (what can be automated) and that that led very quickly tohigh nitrate rates.
I will explain here the two original methods which I developed and of which the use could be spread quickly.
1° the autotrophic denitratation on sulphur
A article published on the site MARCH the 18/5/98 and written by Christophe SOLER summarizes the method rather well. I will thus be satisfied simply to specify the history and to add some information. It is my former professor Guy Martin of the Higher National School of Rennes which is at the origin of this idea; it applied it to the soft water treatment intended for the network of adduction.
From the 1991 I tested this sea water method what was new especially that we did not know if it would have consequences in terms of toxicity with respect to the species present.
It is only fine 94 which, extremely 3 years of experiments without apparent toxicity on many vats and species present at my residence, I proposed this Michel Hignette method preserving of the aquarium of the MAAO. A pilot was launched there by my care.
Since then, the experimentation was carried out on a large scale, as well with the MAAO as with the Large Aquarium of Saint-Malo from which I ensured the technical and scientific direction on June first, 1996 mid-December 1997.
I make a point of specifying that I use since 1996 of sulphur in ball of average diameter 3,5 millimetres marketed by SESOLàSt Herblain(44). This presentation is much easier to use than to start from sulphur out of bar and to crush it with the hammer.
The quantity of sulphur to be used is a function of the initial nitrate rate at the time of the startup and the quantity of food brought. I consider that a volume of sulphur equal to 1% of the total volume of water to be treated is sufficient when the initial nitrate rate is lower than 50 mg/l (NO3 -).
The water flow which can cross the sulphur column depends on the nitrate rate of water to treat: more there is nitrate plus the flow in entry must be weak or else you will find a part of nitrates at exit of column.
You can count for starting on an order of magnitude of 1 L per hour and liter of sulphur in the column. You will adjust then in the following way:
- For a too low flow you will have an egg odor rotted at exit of column due to a production of sulfurous hydrogen (H2S) what will seldom arrive and for very low flows really.
- For a too strong flow you will detect nitrites or nitrates in the water of exit whereas regulated well you can obtain 0 mg/l nitrate at exit.
However the experiment shows that the system is very tolerant as for the output control, flow which will be able to go up to five liters hour per liter of sulphur.
- the water sent on the column can come from a derivation of the aerobic filter or directly of the vat. The column must allow the exhaust of produced nitrogen: for this reason a vertical water circulation upwards appears preferable to me with output control in entry of column and not at exit. The exit of column can be opened with the free air. Water leaving the very acid column of sulphur perhaps but according to my experiments a degasification of this water (for example by injection of air using a diffuser) makes it possible to find a pH close to that of entry. The acidity of this water is thus at least mainly related to the presence of carbon dioxide; from where the idea to use this water to produce an engine with limestone by making it pass in one second (and even a third) column identical in the face to that containing sulphur but this time filled of maërl or coral sand of low granulometry (same sand as that which you use to furnish the bottom with your vat).
This water also contains sulphates in quantity slightly higher than the rate in entry but it was never observed of consequence in 7 years of experimentation even on vats not undergoing any water change during years. It is necessary to say on this subject which an error slipped into the publication made jointly with the MAAO: the sulphur rate present in natural sea water is of almost 900mg/l; this sulphur is present in the form of sulphate (SO4- -)soit approximately 2,65 sulphate g/l, quantity which with it only can explain why the contribution of the sulphate system will be without notable consequence.
2° the Jaubert method doped with sulphur.
The Jaubert method is, now, better and better known; and I use it since 1994. It consists in carrying out the reduction of nitrogen nitrates and the engine with limestone directly in the aquarium. The reduction occurs in the low part of a thick layer of sand from 8 to 10 cm (according to personal communication of J Jaubert). This method functions very well; nevertheless, it seems limited to the fish aquariums not very charged or rather little nourished, probably for lack of matter organic in the low part of sand factor limiting the development of the anaerobic bacteria which transform nitrates nitrogenizes some by nourishing carbonaceous compounds (organic matter).
It also seems that only the quite enlightened aquariums can function according to this method without one having today indisputable explanation on this subject.
Lastly, it is necessary that the surface of the ground of the aquarium which is covered by the decoration does not exceed approximately 25 % (always according to the information communicated by J.Jaubert).
If, for a reason or another, of nitrates are present in a persistent way in water, one can always dope the system to make them disappear, more quickly, by accelerating the bacterial process by organic matter introduction into the low layer of sand (glucose for example introduced by a cane penetrating under the layer and emerging above the surface of the water of the aquarium).
To make function this Jaubert method beyond its limits i.e. in a very nourished and little or not lit aquarium, without having to add glucose under the layer, while preserving its advantages to know extreme simplicity, not from external engine, not from flow to be regulated, engine with limestone to incorporate, etc the idea came to me, a few years ago, to free me from the limiting factor which the quantity of organic matter constitutes present in the low part of sand by doping the system with a small layer of sulphur in balls placed at this level. This makes it possible to make function the system in an autotrophic carbon way and either only heterotrophic.
This device that I tried out successfully consists of a traditional basic grid style filters under sand but without suction pipe (water crosses the ballasting only by natural diffusion between the sand grains as in the Jaubert method).
The experimental protocol that I set up at the end of August 1998 was as follows:
I have completely remakes an aquarium of 100 liters which had turned for several years and which had 6 to 8 cm of coral sand and the alive rocks. It should be noted that this vat, although installed according to principles' of the Jaubert method, saw its rate nitrate to go up for the periods of nourrissage.
Of the same vacuum of any animal and not nourished during months its rate of nitrate did not drop. It is the perfect of a vat Jaubert little or not lit example and which does not function sufficiently quickly, undoubtedly for lack of nutrients for the anaerobic bacteria present in the layer.
I went up the whole while intercalating between the basic grid and the ballasting a layer of sulphur in balls of approximately 0,5 cm thickness placed between two layers of plastic mosquito net netting.
The 65 to 70 liters of sea water of the vat had 35 mg/l nitrate(NO3 -). I employed again this water to fill the vat. Five days later, the vat showed a nitrite rate of a value higher than 10 mg/l. Sand used being alive sand, already sown in aerobic bacteria, it is the first stage of the anaerobic transformation of nitrite nitrates which was occurring on sulphur and not the transformation of ammonia into nitrite like traditionally in a vat lately installed.
The tenth day, the nitrite rate was about 1 Mg per liter; the transformation of nitrites into gas nitrogen was thus in hand. But in a system of this type, it is logical that the water circulation occurring by natural diffusion through the ballasting the evolutions measured in the water of the vat are slower than those noted on the outlet side of an engine with sulphur where one forces water to cross the filter.
The eleventh day, there did not remain any more nitrite and the rate of nitrate had fallen to 5 mg/l.
The twelfth day, the nitrates were not detectable any more whole (lower than 1mg/l).
With the eightieth day the vat is always free from nitrate although it receives each day a cube of artémias frozen of approximately 1 Cm3 what is high a enough contribution for 65 liters of water. The tubastréa auréa present in this vat for 2 months has been practically open all the day appreciating the fort running which reigns in the vat and the daily contribution of food. It is the same for the actinias équina and the two specimens of carotalcyon sagamianum. The pH is about 7,7 because of the presence of carbon dioxide (a degasification makes go up the pH) but that does not seem to obstruct the invertebrates. KH lies between 6 and 7. It is also that of the water of the English Channel which was used at the origin to fill this vat.
This method, very interesting, could perfectly installation in the additional decantation of an existing vat of the kind that one is not obliged to remake the whole of the vat. The relatively low pH surely will reject some among you, but it should be known that with the Jaubert method, the pH and KH are in general definitely low than with the Berliner method (at least the morning for the pH). It is without notable consequence on the animals. My vat is not enlightened (if it is not by the light of the part) it is not ventilated differently than by a normal exchange with the interface air-water, this interface being brewed little besides since I privileged the current on the corals in order to bring their food to them.
In an enlightened and brewed aquarium surfaces some or having diffuser, carbon dioxide dissolves will be eliminated better and the pH will be surely higher the more so as it is undoubtedly not necessary to have sulphur on all the surface of the vat such as I did it.
I am persuaded for my part that this presence of CO2 in quantity in water is not inevitably a problem but the future will say it to us. As soon as I can acquire some I add in this aquarium some Dendronephtyas, ombrophilous gorgones, Sphaerella krempfis, sponges etc.... the food as for it must be able to be automated and thus more regular using slot-machine bus of simple flakes for fish are enough to make open Tubastréa auréa.
Thank you to make me share of your experiments in this direction.
To follow.
Marc LANGOUET.
6/11/98
NB 1
Autotrophic:qui does not require the contribution of an energy or nutrients external with the system.
Heterotrophic: who requires it
NB 2 You can make share of your comments or of the results of your experiments is directly with
Marc LANGOUET
Guimorais
35350 St Coulomb
tél.+fax. 02 99 89 41 69
Maybe with the regional contact of Reef France in Brittany which will transmit
Pierre ZMIRO
17 street of the station
49440 Candé
Tel.. 02 41 94 92 10
Fax 02 41 94 92 11
FBZ@wanadoo.fr E-mail
-------------------------------------------------------------
Various questions, Answers & Infos
Q : Degasification (exhaust of produced nitrogen) is evoked by Marc in his article. Not having decantation in my vat, I want to place the column in bottom in the piece of furniture; it will be thus completely full of water. I add that I will fill the column of sulphur and Maërl with 50/50%. I intend to reject water to treat with the free air with the top of the water level of the vat, but will that be enough for degasification? Can I function thus without risk while being also effective?
R of Marc: There is no problem so that the column is full of water; It is necessary that circulation is done upwards in the column sulphur /maërl, but it should be noted that it because the maërl will be reduced out of mashed potaties with time and will have to be thrown the whole is not a very good idea because that will be clogged... Better is worth to separate sulphur and sand because sulphur can be preserved: it does not worsen.
Q : Should sulphur be added regularly?
R of Marc: There is a sulphur consumption but it is very weak it corresponds to the transformation:
4NO3 + 3S = 2 N2 + 3 SO4
(that is more complex but the assessment returns to that)
Actually I did not add sulphur during years nor even less changed sulphur.
Q : With what is used exactly the air intake and it is fundamental?
R of Marc: The air intake is not there that to facilitate the exhaust of nitrogen. If one works on nitrate rates enough élevés.Mais if the nitrate rate in entry of the engine is weak this nitrogen degasification will be weak and I think that it will not pose problem to send this water in an Visio-engine but the experiment remains to be made.
Q : Which is the cost of Sulphur?
R of Marc: The cost of sulphur is about 10 to 30 FF/kg
Q : I made an engine according to articles' of MARCH with 2 columns of 5 liters; a container of the sulphur balls, and the other containing a calcareous substrate standard engine with limestone gross granulometry. In eight day, the nitrates which were approximately 25 mg/l in my vat of 400 liters, were found reduced to Zero at exit of column. Since, approximately one month after its startup, the nitrate rate ranges between 0 and 5 mg/l in the vat. It is really effective!!
But as anything is not easy in récifal, it exists other parameters quite as important, which can be, them also, touched by the engine with sulphur. For calcium first of all, I had 410 mg/l approximately at the beginning, and all
continuously as usual my contribution by lime water for evaporation (5 liters per week approximately because my closed vat evaporates little, recall: lighting exclusively in fluos). I have at the end of one month 490 mg/l at exit of column!! It, do I have is brilliant to suppose that it is the limestone column which makes effect?
On the other hand, the pH, is to him since the beginning lower by 0,6 to 0,8 at exit of column compared to the vat. Thus, it is descended in the vat from 8,2 to 7,8 at the end of one month (7,05 at exit of column). What can I make to increase the pH without upsetting properly the animals? This fall of pH arrètera-you it one day? This problem is it inherent with the systême of denitratation on sulphur and thus that to make to stabilize the pH in the surrounding of 8,3/8,4?
R of MARCH: The flow of the engine is not indicated. Perhaps can one play above (with the rise or the fall) in order to reduce the amount of acid distributed in the aquarium, to test by measuring the pH of exit, as for an engine with limestone. Another possibility: Always as in an engine with limestone, Si the sand of coral is too large, water passes too quickly inside, with a heat-transferring surface miniumum, which does not make it possible to dissolve sand enough and thus to reduce the acidity of water. For remèdier with that, that is to say to increase the length of the circuit in the coral sand, or more simply to take sand of granulomètrie between 1 and 5 mm.
Q : How gèrer production of nitrites at the time of the launching of the engine?
R of Marc: One should not especially work has flow raised at the beginning during the launching phase because the reaction is anaerobic (without oxygen), and thus if you work with high flow there will not be average to pass in anaerobiose and the reaction will not start. On the contrary it is necessary to reduce the flow to lowest possible (of the order of a drop for the second for 1 sulphur L, and thus the produced nitrites can be sent in the vat without risk: sight their small quantity they will be transformed by the aerobic filtration of the vat into nitrates. At the end of 3 to 5 days the nitrites will disappear from water at exit and you will be able to increase the flow until reaching gradually the cruising speed while taking care during this phase of growth not to make reappear the nitrites by a flow too fast Les nitrates will fall them have zero as soon as the nitrites disappear and have condition of not too quickly increasing too much nor the flow; There is no water change to make. The quantity of nitrites at the time of starting is related to the quantity of nitrates in the water of the vat: if the nitrates are weak, the nitrites will be it too.
Q : Is it necessary to take special precautions when one stops an engine with Soufre?
R of C Soler: If you stop the denitrator by leaving this one filled, it is necessary to let run several times its volume for the re-starting (hydrogen sulphide), you will see that really been able. When the water which leaves the engine does not feel any more you can let it run again in the vat.
--------------------------------------------------------------------------------
Warning of MARCH :
1 the use of this type of system, after one year of experiment in many aquarists, seems always promising, but generates some perverse effects that it is necessary for us to learn with maitriser:
- KH drops
- calcium surdose important (800 mg/l by ex, but any excess is harmful)
- various difficulties
If the fall of the nitrate rate is obvious, remains some details to be developed. MARCH hopes to be in measurement to publish soon the synthesis of various experiments on the subject
-----------
Last edited:


