Bpb
New member
I'll try to be as brief as possible, as I tend to get long winded. Everyone wants full tank stats and history before diagnosing anything so here goes.
90 gallon display very rock heavy
40 gallon sump all 3 chambers filled with rock and macro algae
2x250 watt Radiums in lumenmax 2 reflectors, m80 ballasts
48" BML super actinic led strip
WP40 and MP40 for flow
Jaebo DC return pump
2 part dosing on BRS pumps through apex, hourly
78-80 degree temp range, usually doesnt drift far beyond 78.5
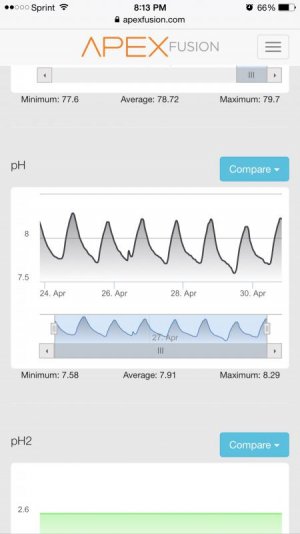
Alk: 7.0 dKH on average,
Ca: 450 ppm
Mg: 1450 ppm
PO4: 0.04 ppm
NO3: 1 ppm
Age: 11 months old
5% water change weekly, 10% at the first of each month using standard IO
all RODI for ATO and water changes, membrane & DI resin replaced 2 months ago, always producing zero tds, via the HM dual meter which I calibrate monthly
SG: 1.026 via the VeeGee seawater refractometer which I also calibrate monthly (though it never drifts, I suppose I CHECK it monthly)
Food: BRS Reef Chili and Cyclopeeze target fed to all corals 3x a week
PE Mysis and Elos Amino acids broadcast fed to fish 3-4x a week
NLS pellets fed to fish daily 2-3 times
On my previous tank I had fantastic success growing any acropora I dropped in the water. It was miraculous how fast everything grew. I also ran an oversized protein skimmer, biopellet reactor, GFO reactor, and rox 0.8 carbon in a reactor. My colors and growth were tremendous. I shut the tank down at the 2 year mark and upgraded to my 90 gallon. I kept all of my old rock and it was an instant "day-of" transfer of tank and corals" I also added a good bit of pukani and aquamaxx dry rock as well, which was well rinsed and soaked in RODI, as well as cycled on it's own in a remote container.
A couple months prior to breaking down my old tank I started getting some STN on many of my acropora. I attributed it to excessive chemical filtration and too low of nutrients because my colors became pale also. No bite marks or signs of predation or pests, and PE was still excellent all around. I fragged up most of my colonies before transfering and sold them to several friends. Those colonies are now flourishing in all of their tanks a year later.
After tank transfer I decided to cease biopellets and let my nutrients build up a bit in the tank to try and encourage some Zoa and LPS growth, which worked. Over the months due to test results I also discontinued GFO and carbon as well, being that I didnt have a tremendous nuisance algae problem and my NO3 and PO4 always came in undetectable. After 6 months or so with my current tank I started to re-introduce acropora, which I had NONE of left upon tank transfer.
I started with frags of my old colonies to see how they would do. Within a few weeks all STN and died entirely, despite every effort to save them via fragging and feeding. Since then for the past 6 months it has been more of the same. I've tried over and over buying frag pack after frag pack of acropora, only to watch them all STN and die within a few weeks of going in the water.
Acropora have been my passion in the hobby since I started and it's been impossible for me to stomach not keeping them and going another direction. To date I've lost probably 30 unique acropora frags I've attempted in the past 6 months, with only 4 remaining alive, and growing very very very slowly. Those who seem to survive are an acropora hyacinthus, ora hawkins echinata, purple acropora meridiana, and tricolor valida.
I test religiously and am very particular about not putting my hands in the tank more than necessary, strict dipping policy for all corals, and really focusing on stability. Yet no matter what I do, or don't do, Acropora specifically just seem to die so quickly for me.
Montipora, LPS, Stylos, Pocillopora, Zoas, all grow exceptionally well. I frag my montipora colonies monthly just theraputically to keep them from taking over too much space, I can barely keep up. I'm increasing my 2 part dosing almost weekly just to meet their demand. Maxima clam doing well too.
I have a friend getting out of the hobby who I'm going to pick up several acros from. I havent tried any new acros in a few months now because I've been afraid to, but I'm so dissatisfied with my tank as it is. Any ideas on what could possibly be causing my grief and what I can do to maybe succeed better this go around? I have a newborn in the house and wife is in school right now so time limits me from doing what I really want to do which is shut the tank down entirely, nuke the rock, sell the tank itself, buy a new one, and startover.
Ideas?
90 gallon display very rock heavy
40 gallon sump all 3 chambers filled with rock and macro algae
2x250 watt Radiums in lumenmax 2 reflectors, m80 ballasts
48" BML super actinic led strip
WP40 and MP40 for flow
Jaebo DC return pump
2 part dosing on BRS pumps through apex, hourly
78-80 degree temp range, usually doesnt drift far beyond 78.5
Alk: 7.0 dKH on average,
Ca: 450 ppm
Mg: 1450 ppm
PO4: 0.04 ppm
NO3: 1 ppm
Age: 11 months old
5% water change weekly, 10% at the first of each month using standard IO
all RODI for ATO and water changes, membrane & DI resin replaced 2 months ago, always producing zero tds, via the HM dual meter which I calibrate monthly
SG: 1.026 via the VeeGee seawater refractometer which I also calibrate monthly (though it never drifts, I suppose I CHECK it monthly)
Food: BRS Reef Chili and Cyclopeeze target fed to all corals 3x a week
PE Mysis and Elos Amino acids broadcast fed to fish 3-4x a week
NLS pellets fed to fish daily 2-3 times
On my previous tank I had fantastic success growing any acropora I dropped in the water. It was miraculous how fast everything grew. I also ran an oversized protein skimmer, biopellet reactor, GFO reactor, and rox 0.8 carbon in a reactor. My colors and growth were tremendous. I shut the tank down at the 2 year mark and upgraded to my 90 gallon. I kept all of my old rock and it was an instant "day-of" transfer of tank and corals" I also added a good bit of pukani and aquamaxx dry rock as well, which was well rinsed and soaked in RODI, as well as cycled on it's own in a remote container.
A couple months prior to breaking down my old tank I started getting some STN on many of my acropora. I attributed it to excessive chemical filtration and too low of nutrients because my colors became pale also. No bite marks or signs of predation or pests, and PE was still excellent all around. I fragged up most of my colonies before transfering and sold them to several friends. Those colonies are now flourishing in all of their tanks a year later.
After tank transfer I decided to cease biopellets and let my nutrients build up a bit in the tank to try and encourage some Zoa and LPS growth, which worked. Over the months due to test results I also discontinued GFO and carbon as well, being that I didnt have a tremendous nuisance algae problem and my NO3 and PO4 always came in undetectable. After 6 months or so with my current tank I started to re-introduce acropora, which I had NONE of left upon tank transfer.
I started with frags of my old colonies to see how they would do. Within a few weeks all STN and died entirely, despite every effort to save them via fragging and feeding. Since then for the past 6 months it has been more of the same. I've tried over and over buying frag pack after frag pack of acropora, only to watch them all STN and die within a few weeks of going in the water.
Acropora have been my passion in the hobby since I started and it's been impossible for me to stomach not keeping them and going another direction. To date I've lost probably 30 unique acropora frags I've attempted in the past 6 months, with only 4 remaining alive, and growing very very very slowly. Those who seem to survive are an acropora hyacinthus, ora hawkins echinata, purple acropora meridiana, and tricolor valida.
I test religiously and am very particular about not putting my hands in the tank more than necessary, strict dipping policy for all corals, and really focusing on stability. Yet no matter what I do, or don't do, Acropora specifically just seem to die so quickly for me.
Montipora, LPS, Stylos, Pocillopora, Zoas, all grow exceptionally well. I frag my montipora colonies monthly just theraputically to keep them from taking over too much space, I can barely keep up. I'm increasing my 2 part dosing almost weekly just to meet their demand. Maxima clam doing well too.
I have a friend getting out of the hobby who I'm going to pick up several acros from. I havent tried any new acros in a few months now because I've been afraid to, but I'm so dissatisfied with my tank as it is. Any ideas on what could possibly be causing my grief and what I can do to maybe succeed better this go around? I have a newborn in the house and wife is in school right now so time limits me from doing what I really want to do which is shut the tank down entirely, nuke the rock, sell the tank itself, buy a new one, and startover.
Ideas?