You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Donovan's Nitrate Destroyer
- Thread starter djbon
- Start date
but most experienced reefer will tell you that anaerobic bacteria can be found only a few milimeter off the porous rocks, provided that the outer layer are slime coated by aerobic bacteria. from #381.
I seriously doubt that more that a few experienced reefers have ever looked at the distribution of aneorbic bacteria on or in anything in their lives. They read that somewhere and pass it on as "truth", but in reality virtually every reefer has no idea of the distribution of anaerobic bacteria in their system. I don't either.
Name me a single experienced reefer that has ever examined the distribution of anerobic bacteria (or even aerobic bacteria) in or on anything that is in their reef tank. It is not an easy thing to do!
I seriously doubt that more that a few experienced reefers have ever looked at the distribution of aneorbic bacteria on or in anything in their lives. They read that somewhere and pass it on as "truth", but in reality virtually every reefer has no idea of the distribution of anaerobic bacteria in their system. I don't either.
Name me a single experienced reefer that has ever examined the distribution of anerobic bacteria (or even aerobic bacteria) in or on anything that is in their reef tank. It is not an easy thing to do!
djbon
New member
I am not in the position to rubbish such claim either. I am an engineer by profession, so all about reefing is definitely via what I read on the internet. I came across quite a number of posting of successful denitrification by simply dumping denitrification bio balls in the sump. Assuming the volume is correct for extremely high running water, proportionally a smaller size of the same material should perform on par in a much smaller amount of running water, don't you agree?
djbon
New member
I have been asking myself, if media size does play a role for successful denitrification in a reactor, how about sulfur reactor?. Volume doesn't seem applicable here, let alone sizes. What about bio pellets, is bacteria do lives in the pellets itself?. Size doesn't seems to fit the criteria as well. I do believe oxygen is the key here. All these reactors depends on oxygen deprived condition for denitrification to happen.
We assume that when someone posts that dumping bioballs in the sump led to a reduction in nitrates, that it was the addition of the bioballs in the sump that caused the drop in nitrates, but it need not be. People often add things like that when things aren't going well, and often make multiple changes simultaneously.
the nice thing about a device like yours is you can measure in and out, so you know the denitrification is taking place in the chamber.
Without experiments such are running multiple parallel reactors with different flows, or different media, I think we are just making guesses- hopefully educated, but probably in part made based on claims that are baseless.
the nice thing about a device like yours is you can measure in and out, so you know the denitrification is taking place in the chamber.
Without experiments such are running multiple parallel reactors with different flows, or different media, I think we are just making guesses- hopefully educated, but probably in part made based on claims that are baseless.
Let put one more variable....since I post the foto ,showing for first time that my reactor actually denitrify, I took two more no3 .measurement since then. First with no different value of no3 between DT and reactor and the second two days ago, with similar than the earlier foto results! The only thing I change, is that I still I increasing dosage of diy nopox slowly. I am currently dose 12ml splited in two dosages 8:30 & 18:30. I can not understand d why I can not take constant results from the reactor. I expected once I take measurements, that indicate , denitrification occur inside the reactor, that the results will be every time better, increasing the difference of no3 between DT and reactor.....but that didn't happen!
djbon
New member
We assume that when someone posts that dumping bioballs in the sump led to a reduction in nitrates, that it was the addition of the bioballs in the sump that caused the drop in nitrates, but it need not be. People often add things like that when things aren't going well, and often make multiple changes simultaneously.
the nice thing about a device like yours is you can measure in and out, so you know the denitrification is taking place in the chamber.
Without experiments such are running multiple parallel reactors with different flows, or different media, I think we are just making guesses- hopefully educated, but probably in part made based on claims that are baseless.
I would like to try all bio balls and rings on my next build and see how it performs. For much bigger reactor, coarser media seems to be ideal to prevent clogging due to massive bacteria build up (4" diameter pipe or bigger). Theoretically it should perform much better as anaerobic and aerobic area presents all over the reactor.
djbon
New member
Let put one more variable....since I post the foto ,showing for first time that my reactor actually denitrify, I took two more no3 .measurement since then. First with no different value of no3 between DT and reactor and the second two days ago, with similar than the earlier foto results! The only thing I change, is that I still I increasing dosage of diy nopox slowly. I am currently dose 12ml splited in two dosages 8:30 & 18:30. I can not understand d why I can not take constant results from the reactor. I expected once I take measurements, that indicate , denitrification occur inside the reactor, that the results will be every time better, increasing the difference of no3 between DT and reactor.....but that didn't happen!
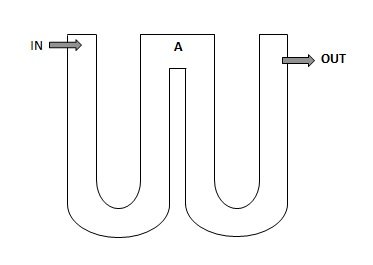
I have no idea what is happening in your reactor but I do feel that for a longer chambers like yours, bacteria die off might be the culprit. Maybe some bacteria died on the way out. It it possible to add a NO3 sampling outlet at "A" as per attached picture?. Another possible reason for your case could be due to no skimmer/mechanical filter to remove excess bacteria. I caught all excess bacteria (excessive bacteria slime on socks) to be washed off the sink, but I am no longer doing it as all excess bacteria are now being consumed by corals (skimmer runs for 9 hours at night and needed to be cleaned every 5 days).
Attachments
blackthunda77
New member
Hey guys. I visited the thread a couple months ago and was interested in the build. I decided to go with biopellets since I had all the materials on hand. Well, I'm still battling no3 so I think I want to try my hand at this. Any recommendations for what size pvc to use? It's a 200 Gal display tank with 80 gal sump. Roughly 220 gals total volume I would say.
Sent from my SAMSUNG-SM-N920A using Tapatalk
Sent from my SAMSUNG-SM-N920A using Tapatalk
I am currently testing a reactor that is all lavarock (cheap pumice-like material around size of 1.5-2.5 cm rocks. To do this, instead of using an aquarium, I am simply using a 40 liter bucket with salt water with nitrates at about 50 ppm from a water change from my regular system, a heater and a doser that puts in two 1.5 ml aliquots of NOPOX per day. I am going to let it run one month and see what happens.
In my 500 gal system that has the high nitrates I am trying a reactor that is 4 inch in diameter and filled with 90% "large" pumice similar to matrix and 10% old Eheim ceramic rings I had lying around. I am dosing that with NOPOX ( 70% 5% vinegar, 30% vodka) 1 ml every two hours. Flow is around 8 liters per hour ~ 200 liters per day- or turning over tank in the reactor 1 every 10 days. This assumes the reactor is working perfectly and all outflow is 0 ppm.
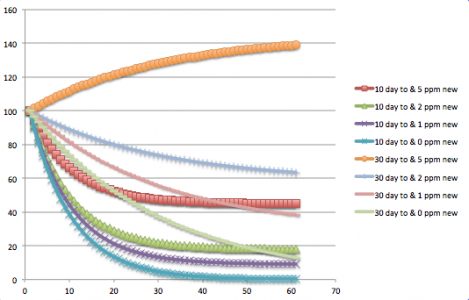
The reason I chose to turn over every 10 days, is because once you get much past that it takes a very long time to lower nitrates (especially if there is any new nitrate production). see attached chart. which shows [ ] of nitrates in tank if flow of tank volume through the reactor is 1 per 10 days or 1 per 30 days. and daily nitrate production is 0, 1ppm, 2ppm, or 5ppm.
I have not inoculated any of these reactors with bacterial stocks. My philosophy on that is that eventually, what type of bacteria can sustainably colonize the reactor under the type of conditions we run it will eventually dominate. So, I figure I will let it colonize naturally (even if it may take a few additional weeks to get started.
In my 500 gal system that has the high nitrates I am trying a reactor that is 4 inch in diameter and filled with 90% "large" pumice similar to matrix and 10% old Eheim ceramic rings I had lying around. I am dosing that with NOPOX ( 70% 5% vinegar, 30% vodka) 1 ml every two hours. Flow is around 8 liters per hour ~ 200 liters per day- or turning over tank in the reactor 1 every 10 days. This assumes the reactor is working perfectly and all outflow is 0 ppm.
The reason I chose to turn over every 10 days, is because once you get much past that it takes a very long time to lower nitrates (especially if there is any new nitrate production). see attached chart. which shows [ ] of nitrates in tank if flow of tank volume through the reactor is 1 per 10 days or 1 per 30 days. and daily nitrate production is 0, 1ppm, 2ppm, or 5ppm.
I have not inoculated any of these reactors with bacterial stocks. My philosophy on that is that eventually, what type of bacteria can sustainably colonize the reactor under the type of conditions we run it will eventually dominate. So, I figure I will let it colonize naturally (even if it may take a few additional weeks to get started.
Attachments
Last edited:
djbon
New member
I dosed sodium nitrate in my DT and brought up NO3 to 20ppm. I didn't change reactor outflow but tripled my carbon dosing daily (3ml x 3) and within 4 days outflow reduced to trickling (maybe 3 drops per seconds) and overflowed my reactor. NO3 is back to 3ppm in 7 days after the overflowing happens (carbon dosing back to normal of 3ml soon after overflowed). I will keep doing this for a few times so that I can keep extra data how to run this reactor for cases of nutrient spike.
djbon
New member
Hey guys. I visited the thread a couple months ago and was interested in the build. I decided to go with biopellets since I had all the materials on hand. Well, I'm still battling no3 so I think I want to try my hand at this. Any recommendations for what size pvc to use? It's a 200 Gal display tank with 80 gal sump. Roughly 220 gals total volume I would say.
Sent from my SAMSUNG-SM-N920A using Tapatalk
4" pipe is suitable. Make sure you varies the media sizes, mixture of ping pong size bio ball and ceramic rings, crushed corals of various sizes and pumice rocks at the end of the reactor will perform better. Good luck and do share your experiences
djbon
New member
I am currently testing a reactor that is all lavarock (cheap pumice-like material around size of 1.5-2.5 cm rocks. To do this, instead of using an aquarium, I am simply using a 40 liter bucket with salt water with nitrates at about 50 ppm from a water change from my regular system, a heater and a doser that puts in two 1.5 ml aliquots of NOPOX per day. I am going to let it run one month and see what happens.
In my 500 gal system that has the high nitrates I am trying a reactor that is 4 inch in diameter and filled with 90% "large" pumice similar to matrix and 10% old Eheim ceramic rings I had lying around. I am dosing that with NOPOX ( 70% 5% vinegar, 30% vodka) 1 ml every two hours. Flow is around 8 liters per hour ~ 200 liters per day- or turning over tank in the reactor 1 every 10 days. This assumes the reactor is working perfectly and all outflow is 0 ppm.
The reason I chose to turn over every 10 days, is because once you get much past that it takes a very long time to lower nitrates (especially if there is any new nitrate production). see attached chart. which shows [ ] of nitrates in tank if flow of tank volume through the reactor is 1 per 10 days or 1 per 30 days. and daily nitrate production is 0, 1ppm, 2ppm, or 5ppm.
I have not inoculated any of these reactors with bacterial stocks. My philosophy on that is that eventually, what type of bacteria can sustainably colonize the reactor under the type of conditions we run it will eventually dominate. So, I figure I will let it colonize naturally (even if it may take a few additional weeks to get started.
I am glad somebody is trying to do it in more scientific way. Do share your results please. Thanks.
blackthunda77
New member
Thanks,4" pipe is suitable. Make sure you varies the media sizes, mixture of ping pong size bio ball and ceramic rings, crushed corals of various sizes and pumice rocks at the end of the reactor will perform better. Good luck and do share your experiences
Would you say that your illustration on post 71 is still what you would recommend? Also what's the difference between the bio rings in the first chamber and the ceramic rings on the bottom?
Sent from my SAMSUNG-SM-N920A using Tapatalk
djbon
New member
Thanks,
Would you say that your illustration on post 71 is still what you would recommend? Also what's the difference between the bio rings in the first chamber and the ceramic rings on the bottom?
Sent from my SAMSUNG-SM-N920A using Tapatalk
Yes. My first intention of mixing various sizes of media is simply for proper flow actually. Sandwich configuration was the first thing popped up in my mind when I first designed this reactor, and amazingly it works fine with my system. I put ceramic rings at the bottom to ease the flow as the inter-chamber connecting pipe is 1/2" in size. When I revamp my design using double elbow for "U" configuration, I put much bigger media at the bottom. It doesn't really matter where does this media goes to, as long as they are sandwich together.
djbon
New member
my experiments would get a solid D- for experimental controls etc.
I wish I could really set up the proper controlled experiments.
But that is for when I retire from my day job as a molecular biologist.
At least you have the experience up to a molecular level. The only thing I know is inches and millimeters which is clearly visible on measuring tapes
djbon
New member
Nitrogen bubbles coming out from the effluent outlet. This unit start producing nitrogen bubbles on the 3rd day and lasted for few days. But his NO3 is not that bad, started at 25ppm and reduced to almost zero in 3 weeks. 3 footer tank of LPS and shrooms.
Attachments
blackthunda77
New member
Do you think 3" pipe would be sufficient? I went to the hardware store and looked at the 4" pipe. Those things are HUUUGE! Lol4" pipe is suitable. Make sure you varies the media sizes, mixture of ping pong size bio ball and ceramic rings, crushed corals of various sizes and pumice rocks at the end of the reactor will perform better. Good luck and do share your experiences
Sent from my SAMSUNG-SM-N920A using Tapatalk
Similar threads
- Replies
- 10
- Views
- 524
- Replies
- 0
- Views
- 144
- Replies
- 7
- Views
- 645