You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Flame Angelfish Breeding attempt
- Thread starter fla2341
- Start date
I'm wondering if the toxic copepod that Martin was referring to is Euterpina acutifrons. In the 1980s I was Syd's assistant and if I remember correctly Syd thought that E. acutifrons was somehow toxic to larvae because the larvae died a few days after being fed the E. acutifrons. It is now thought that the larvae were not able to digest this copepod as a first food. I feel that this copepod still has value as a later stage food and I still have it in culture. I'm also a firm believer in parvocalanus. It works well for the first 14 to 20 days (and sometimes longer). I have gotten C. loriculus larvae on to Artemia after parvocalanus around day 16 through day 30 but then lose them to what I think is a bacterial problem. I was using oily enrichments for the Artemia which also is a wonderful food for bacteria....
Wild plankton does seem to show the greatest success for larval rearing of difficult species and I think ciliates are a large part of that but we need to pursue the cultured live feeds to really make larval rearing prove feasible everywhere.
Karen
Wild plankton does seem to show the greatest success for larval rearing of difficult species and I think ciliates are a large part of that but we need to pursue the cultured live feeds to really make larval rearing prove feasible everywhere.
Karen
fla2341
New member
billsreef: Yes interesting stuff. This is what I was looking for, information to the contrary or further information on suitable/not suitable feeds.
Luis A M, GreshamH, paka: Thank you all. This is the type of shared information I was looking for. If/when I complete the cycle for these fishes I would like to write a summary on everything know or suspected, how that information evolved and how we've gotten to that point. I think this type of information who did what, where and when and what the results were will help others in the persuit. If nothing else it will give those who come after a single source for this type of information so they might not spend time on what has proven to fail but concentrate on improving what did work and/or trying new approaches.
Luis A M, GreshamH, paka: Thank you all. This is the type of shared information I was looking for. If/when I complete the cycle for these fishes I would like to write a summary on everything know or suspected, how that information evolved and how we've gotten to that point. I think this type of information who did what, where and when and what the results were will help others in the persuit. If nothing else it will give those who come after a single source for this type of information so they might not spend time on what has proven to fail but concentrate on improving what did work and/or trying new approaches.
fla2341
New member
paka: Do you have any photos of the C. loriculus at different stages up to the 30 day mark? Can you recount the methods you've employed to have gotten to this stage or is there a place you can point me to read this? Treatment/collection of the eggs, prolarvae, larvae, vessel(s) used, aeration/none, used nanno/not etc... I think this would be very informative.
Luis A M
Premium Member
Paka,so you are our famous Karen B.?
Failure to raise larvae is sometimes explained by the lack of some unknown first food organism.I didn´t share that and sustained that virtually all marine larvae could/should feed on copepod nauplii,the size of a Parvo N1,and that our failures were due to environmental reasons.
Now this last achievement of Frank B.with the triggerfish,kicked me back to the other end of my perpetual "Is it the food or is it the environment"dilemma.The trigger could not be raised with copepods good for Centropyge,and could only be done when a key organism,the ciliate Strombidium was offered.
This must encourage researchers with access to wild plankton,to try and isolate new species since perhaps some valuable key organism is still there waiting to be found
Failure to raise larvae is sometimes explained by the lack of some unknown first food organism.I didn´t share that and sustained that virtually all marine larvae could/should feed on copepod nauplii,the size of a Parvo N1,and that our failures were due to environmental reasons.
Now this last achievement of Frank B.with the triggerfish,kicked me back to the other end of my perpetual "Is it the food or is it the environment"dilemma.The trigger could not be raised with copepods good for Centropyge,and could only be done when a key organism,the ciliate Strombidium was offered.
This must encourage researchers with access to wild plankton,to try and isolate new species since perhaps some valuable key organism is still there waiting to be found
fla2341
New member
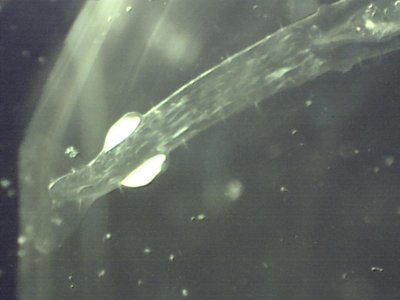
Needlefish larvae? I just found these in the sample which passed thru the 300 micron mesh. They are almost completely clear, have highly reflective eyes and a heart shaped spot on the tail. They measure 4-8mm are long and extreemly thin.
Attachments
Hi Luis, Yes it's me Karen B. It's nice to be communicating with you again.
fla2341- I'm sorry I don't have any photos. I have a camera and adapter for my microscope but I didn't get the correct ones and they don't work well. I was working with the C. loriculus in 2006 and 2007. I did find my notes and will answer your questions hopefully tonight after work.
Karen
fla2341- I'm sorry I don't have any photos. I have a camera and adapter for my microscope but I didn't get the correct ones and they don't work well. I was working with the C. loriculus in 2006 and 2007. I did find my notes and will answer your questions hopefully tonight after work.
Karen
Here are the main points from my notes while I was getting to day 20 fairly consistently and sometimes to day 30 with C. loriculus.
I used a 15 gallon tank with black on 3 sides and a 15 watt cool white bulb about 12 inches above the water. Eggs were collected in the morning from the surface of the broodstock tank using a net and added directly to the rearing tank. I used slow aeration and if the fish looked strong I turned it up and if they looked tippy I turned it down. I used Iso and nanno and culture parcovalanus. I tried to keep the parvo nauplii density between 5 and 10 per ml but keep in mind that with low aeration the nauplii are not evenly distributed so the counts are not truly accurate. By day 4 larval guts should look full and I counted the red spots in the guts as nauplii and having around 8 nauplii was a good sign. I changed at least half of the water every day. Sometimes around day 9 I would add rotifers if I didn't have enough parvo nauplii. Rotifers were enriched with Algamac. I tried to keep rotifers at 0.5/ml. I tested for ammonia every few days and it was 0. I usually had good survival of larvae until around day 16. If the larvae look large enough I added some newly hatched Artemia around day 16 and sometimes a little later. Also used day 2 Artemis enriched. Numbers of larvae would slowly dwindle from here.
The largest most robust deeper bodied larvae often died first. I think they reached a developmental stage that they were not strong enough to get past. I think that better nutrition, perhaps more enrichment would help but that would also mean more frequent water changes with more water volume changed. Maybe your sand bed will help with that.
I hope this helps anyone trying to rear centropyge and if you have any suggestions for me to try I'd love to hear them.
Karen
I used a 15 gallon tank with black on 3 sides and a 15 watt cool white bulb about 12 inches above the water. Eggs were collected in the morning from the surface of the broodstock tank using a net and added directly to the rearing tank. I used slow aeration and if the fish looked strong I turned it up and if they looked tippy I turned it down. I used Iso and nanno and culture parcovalanus. I tried to keep the parvo nauplii density between 5 and 10 per ml but keep in mind that with low aeration the nauplii are not evenly distributed so the counts are not truly accurate. By day 4 larval guts should look full and I counted the red spots in the guts as nauplii and having around 8 nauplii was a good sign. I changed at least half of the water every day. Sometimes around day 9 I would add rotifers if I didn't have enough parvo nauplii. Rotifers were enriched with Algamac. I tried to keep rotifers at 0.5/ml. I tested for ammonia every few days and it was 0. I usually had good survival of larvae until around day 16. If the larvae look large enough I added some newly hatched Artemia around day 16 and sometimes a little later. Also used day 2 Artemis enriched. Numbers of larvae would slowly dwindle from here.
The largest most robust deeper bodied larvae often died first. I think they reached a developmental stage that they were not strong enough to get past. I think that better nutrition, perhaps more enrichment would help but that would also mean more frequent water changes with more water volume changed. Maybe your sand bed will help with that.
I hope this helps anyone trying to rear centropyge and if you have any suggestions for me to try I'd love to hear them.
Karen
racerw
New member
fla2341- I am sorry but I do not have any pics. The success was with wild plankton and was loaded with ciliates.
I was culturing some wild plankton but recently had to chance from using RG Complete to RG nana and lost all cultures 2 days later. Not sure why so will be collecting more plankton soon.
Karen- if you are up for trying again let me know. I lost my female Interrupta about 3-4 months ago and replaced her with a tiny juvi. They are doing the dance nightly so I am thinking a spawn is in the near future.
I was culturing some wild plankton but recently had to chance from using RG Complete to RG nana and lost all cultures 2 days later. Not sure why so will be collecting more plankton soon.
Karen- if you are up for trying again let me know. I lost my female Interrupta about 3-4 months ago and replaced her with a tiny juvi. They are doing the dance nightly so I am thinking a spawn is in the near future.
fla2341
New member
racerw: Thanks, and dang, I really wanted to see your larval fish to see if there were any early morphological differences. Wild plankton from where? Did you identify what else was in the water you collected? What housing/rearing methods did you use? Did you have to use a reduced water temp?
paka/Karen B.: Thank you so much for this information. It should prove invaluable in our attempts as we know what worked to that point. Now all we have to do is try to get beyond that.
My Observations: I have observed that several of the failed prolarval/larval fish had deformities of one type or another ie: missing or deformed pect fins(spinners), mouth developemental deformaties, spines bent/twisted, deficient oil globules, missing eye(s) etc....
I think that what we are seeing a majority of the time in the drop off rate is mearly natural selection taking it's toll. In every batch there will only be so many which will prove out to be developmentally sound thru meta.
In addition I've seen that changing environmental conditions can also have an adverse effect on developing prolarvae ie: water turbulence, pH, temp and salinity.
Your's could have just as easily succumbed to a genetic deficiency. We know so little as yet and there's always more to learn.
Update:
2 nights ago(July 4th) I had a spawn of 1200+(counted 1292 ish) so far I have hundreds of prolarvae floating in the ten gallon.
prolarval/larval Conditions:
Ammonia 0, pH 8.0-8.1, temp 80-81 deg F, salinity 34-35ppt, lights on when sun comes up(6-7am overcast dependant) off at 11 pm. Light provided by sunlight (direct from 1pm - 6pm) and a 18" 15w flour strip 10" from waters surface which is on from 5pm-11pm. I change out approx 2 gallons of the 5 gallon volume every 2-3 days and replace a quart from evap every day via slow drip. I put a very small amount of kalk into the replacement water to bring that waters pH up to 8.2-8.4 (The tank water's pH drops between water changes to 7.6-7.8 if left untreated). I've also introduced slow air bubbling into the larval chamber and fast bubbling in the sump/pump chamber to help facilitate gas exchange and water circulation.
Jungle Pete suggested that I add volume to the system by adding on an auxiliary tank however this option will have to wait as it's not feasible right now.
More as it developes it's about egg time.
paka/Karen B.: Thank you so much for this information. It should prove invaluable in our attempts as we know what worked to that point. Now all we have to do is try to get beyond that.
My Observations: I have observed that several of the failed prolarval/larval fish had deformities of one type or another ie: missing or deformed pect fins(spinners), mouth developemental deformaties, spines bent/twisted, deficient oil globules, missing eye(s) etc....
I think that what we are seeing a majority of the time in the drop off rate is mearly natural selection taking it's toll. In every batch there will only be so many which will prove out to be developmentally sound thru meta.
In addition I've seen that changing environmental conditions can also have an adverse effect on developing prolarvae ie: water turbulence, pH, temp and salinity.
Your's could have just as easily succumbed to a genetic deficiency. We know so little as yet and there's always more to learn.
Update:
2 nights ago(July 4th) I had a spawn of 1200+(counted 1292 ish) so far I have hundreds of prolarvae floating in the ten gallon.
prolarval/larval Conditions:
Ammonia 0, pH 8.0-8.1, temp 80-81 deg F, salinity 34-35ppt, lights on when sun comes up(6-7am overcast dependant) off at 11 pm. Light provided by sunlight (direct from 1pm - 6pm) and a 18" 15w flour strip 10" from waters surface which is on from 5pm-11pm. I change out approx 2 gallons of the 5 gallon volume every 2-3 days and replace a quart from evap every day via slow drip. I put a very small amount of kalk into the replacement water to bring that waters pH up to 8.2-8.4 (The tank water's pH drops between water changes to 7.6-7.8 if left untreated). I've also introduced slow air bubbling into the larval chamber and fast bubbling in the sump/pump chamber to help facilitate gas exchange and water circulation.
Jungle Pete suggested that I add volume to the system by adding on an auxiliary tank however this option will have to wait as it's not feasible right now.
More as it developes it's about egg time.
Eddie/Stacie What is RG Complete and RG Nana? I'm not familiar with those.
You can email me about the interrupta. kbrittai@hawaii.edu
fla234 I haven't checked the pH on my larval tanks and will do that
You can email me about the interrupta. kbrittai@hawaii.edu
fla234 I haven't checked the pH on my larval tanks and will do that
fla2341
New member
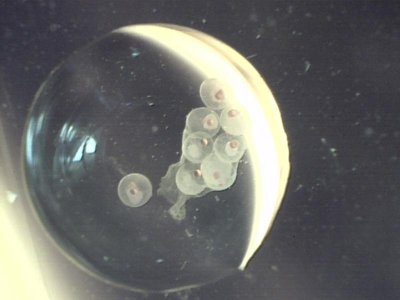
Minor update: Just had an entire collection of eggs turn white or pink with no survivors in the past 4 hours. The last two sets of eggs prior to this I had good hatch outs but the pro larvae almost all died before day 3. They also turned white( the more common color of dead larvae) or pink.
I still have a handfull of larvae from the July 4th batch in the 10 gallon.
I still have a handfull of larvae from the July 4th batch in the 10 gallon.
Attachments
Luis A M
Premium Member
Eddie/Stacie What is RG Complete and RG Nana? I'm not familiar with those.
Karen,they are new products of Reed.Basically NAN,but enriched and/or mixed with other algae.Gresh will probably chime in with more info
Eddie/Stacie What is RG Complete and RG Nana? I'm not familiar with those.
You can email me about the interrupta. kbrittai@hawaii.edu
fla234 I haven't checked the pH on my larval tanks and will do that
Shoot us an email to TechSupport (at) ReedMariculture (dot) com and we'll explain it in full
Gresham H
Reed Maricultue
Karen,they are new products of Reed.Basically NAN,but enriched and/or mixed with other algae.Gresh will probably chime in with more info
Only RGcomplete is new.
RotiGrow Nanno is a few years old.
Luis A M
Premium Member
But returning to the main topic.
Two sides of the problem;the food or the environment.
When prolarvae or pre-feeding larvae die,you know it is the environment.It could be bacteriae or some obscure water chemistry factor.BTW,I think most of the successful reports used NSW
And 1st food organisms deserve more attention,perhaps not so much for Centropyge but for the many other ornamentals that could never be raised.
Chris,you have the conditions to walk that line,collecting and starting lots of gallon culture jars as I described.:beachbum:
Two sides of the problem;the food or the environment.
When prolarvae or pre-feeding larvae die,you know it is the environment.It could be bacteriae or some obscure water chemistry factor.BTW,I think most of the successful reports used NSW
And 1st food organisms deserve more attention,perhaps not so much for Centropyge but for the many other ornamentals that could never be raised.
Chris,you have the conditions to walk that line,collecting and starting lots of gallon culture jars as I described.:beachbum:
glennvandyke
New member
long post to read! but htis is amazing! i been breeding clows for years and looks like im going to get into flames as well! very amazing!
Similar threads
- Replies
- 3
- Views
- 1K
- Replies
- 1
- Views
- 154
- Replies
- 9
- Views
- 1K