i think todays clutch is sixteen.... ah. sweet sixteen. something promising numerologically..and with all those starry alignments, lord xenu , i ask for your blessings... i think. actually i'm just making things up.
sorry if i offended anyone.
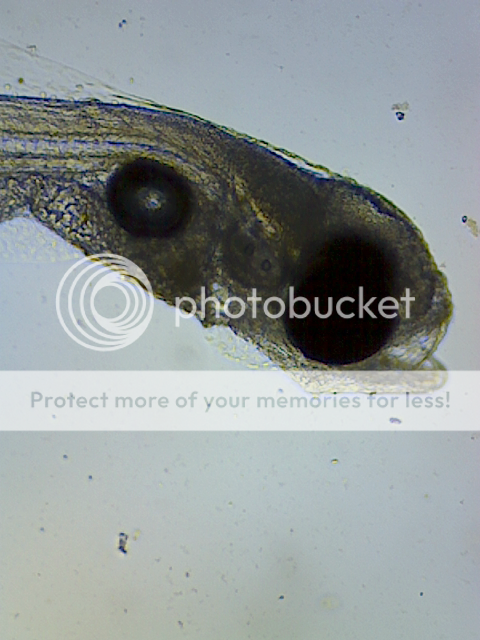
the reason for the sillyness, is that i just had another beer and finished tallying the count of gobie larvae that took me the better part of a great day off planned kicking samurai butt in a good long total war game. i just finished loading my first great battle, and the light flicked on for the dv. i already gave it the flashlight when i woke up. ( i flashlight before i go to bed and when i wake up. ) there was nothing when i glanced over at lights on. the battle on the screen started and my cannons were making short work of their archers. i was winning the assault. but then i neglected to see the sabre cavalry flank my riflemen, and thusly, kill my five star general. so i was a bit peeved and decided to get up and check out the vase. one glance at it and it looks like a snow globe. glittery baby gobies everywhere. no female in sight.
so i quickly quit tw shogun2, and got to work harvesting gobie larvae.
after my last go around a couple of weeks ago, i cleaned out the mulm and scrubbed the algae out of r1, r2 and r3. the crab zoa only made it a few days. the gobies the same. i blame it on the low temps we've had here in southern ontario. i keep everything at room temp, about 76'. it got cooler than that. there was one shrimp zoa left so i just put it in the dv.
i've been catching mysids that have started to overrun my display. they were stocked in the rearing containers, 1,2,and 3. i've learned that keeping the containers completely clean and new is not a good idea. so these seasoned containers come in handy. as in today. i promptly placed the mysids in the 2.6 rv that i've got setup somewhere else with my phyto grow op. there's about a dozen or so of the little buggers. i did catch the female gobie hunting mysids in the dv. just the ones that zipped past her tunnel opening. she's too lazy to chase them any further than two inches. she's really fat. she's been eating the shrimp pellets as well.
so i collected six litres of gobie larvae over the course of the afternoon.
i decided that i'd set up a spread sheet of the different cultures and rearing containers that i was gonna use. i'll post the result of this as i go. with my luck in this endevour, it'll be over before it began.......
this is the result of ten hours worth of work.
the middle shelf has seven one litre containers, each containing between twenty and thirty gobie larvae. either nanno or tetraselmis, dv water, passed through a 56 seive, and five of them only have air bubbles. about one bubble per second. the tall cylinder r-08, is two litre and nocc'd up with tetraselmis. it has air about one per second and fifty gobie larvae. all phyto cultures are seven days from ferts. i avoided using duna other than any stray from rotifer feeds.
(i've gotta coninue this....)