Hey all,
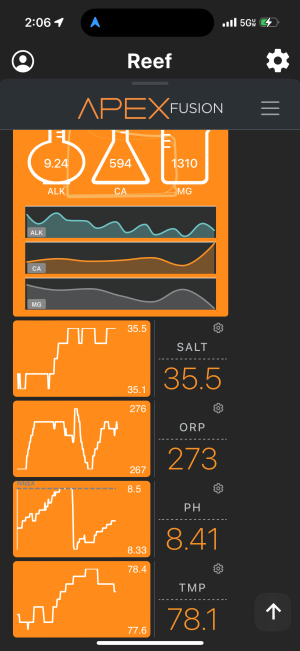
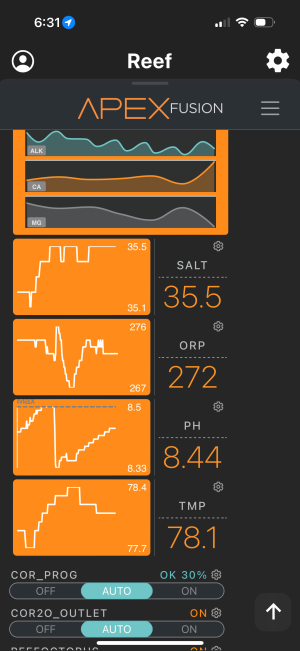
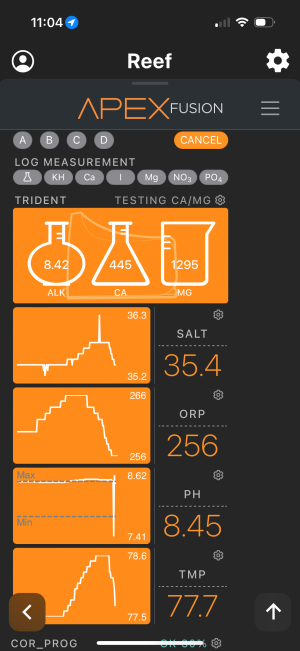
I been battling an ongoing problem in 92 gallon where my ph keeps on climbing up to 8.5 and sometimes more. At 345 this morning I dosed 80ml of distilled vinegar and it dropped from 8.5 to 8.32. As of right now, it is already back up to 8.41 and climbing. I turned off my all dosing and my lights are off. Can anyone assist in what can cause it to continuously climb?
Thank you
I been battling an ongoing problem in 92 gallon where my ph keeps on climbing up to 8.5 and sometimes more. At 345 this morning I dosed 80ml of distilled vinegar and it dropped from 8.5 to 8.32. As of right now, it is already back up to 8.41 and climbing. I turned off my all dosing and my lights are off. Can anyone assist in what can cause it to continuously climb?
Thank you