Now, there are a few ideas from recent posts that have been intriguing me. My reefing skills are a bit rusty from disuse since I've spent the last few years in the dusty halls of academia, but maybe I can say something interesting from that perspective.

First is the discussion of "˜Total Organic Carbon' and its relation to skimmers. Organic carbon in and of itself isn't strictly a problem for our aquaria, but rather the reduced nitrogen and phosphorous that go along with it. The organisms whose growth we're trying to inhibit generally fix their own organic carbon as quickly as they can acquire these limiting elements. Of course we all know this already.
The perspective I'd like to consider is that not all organic carbon is created equally. In the ocean, for instance, there are tremendous pools of really intransigent carbon compounds that get broken down extremely slowly or not at all. These will contribute to TOC but not to algal growth. More labile organic carbon that lacks associated N or P (such as polysaccharides and alcohols) will get consumed by heterotrophic bacteria which we don't care about (e.g. carbon dosing through vodka or whatever). Conversely labile carbon in the form of proteins or phosphorylated fatty acids are going to be more of a problem.
Another really important thing I think about in my research is rate of various processes. For example, most algae aren't going to be directly degrading the problematic nitrogen-containing source organic compounds, they'll be utilizing the byproducts of heterotrophic organisms that take that organic nitrogen and mineralize it into reduced inorganic nitrogen compounds like ammonia and nitrate. This process is comparatively slow. If you export your nitrogen biologically (using macroalgae, DSB, or what have you), you also have to wait for the nitrogen to be mineralized and then compete with the undesirable organisms for it. (The exception of course would be exporting biomass from heterotrophs like sponges). If you can remove the source organic nitrogen using a mechanical or chemical process that operates at a significantly faster rate than the biology, you can get it out of the system before your bad algae even has a chance to compete.
I've read in this thread about some experimental evidence suggesting that skimmers aren't very good at removing TOC. By contrast I've also read a lot of anecdotal evidence that suggests skimming might be pretty good at maintaining water chemistry that keeps algal growth in control. Although these observations might seem to be in tension, it could be that skimmers are very good at rapidly removing the 20% of TOC that is really problematic (also contains organic N and P) before it has a chance of entering the slower biological cycle. And maybe GAC is good at eventually adsorbing a lot of TOC, but it can't do it until that carbon is solubilized, and perhaps by that point you've already mineralized some of the N and P.
I don't really know. Something to consider!
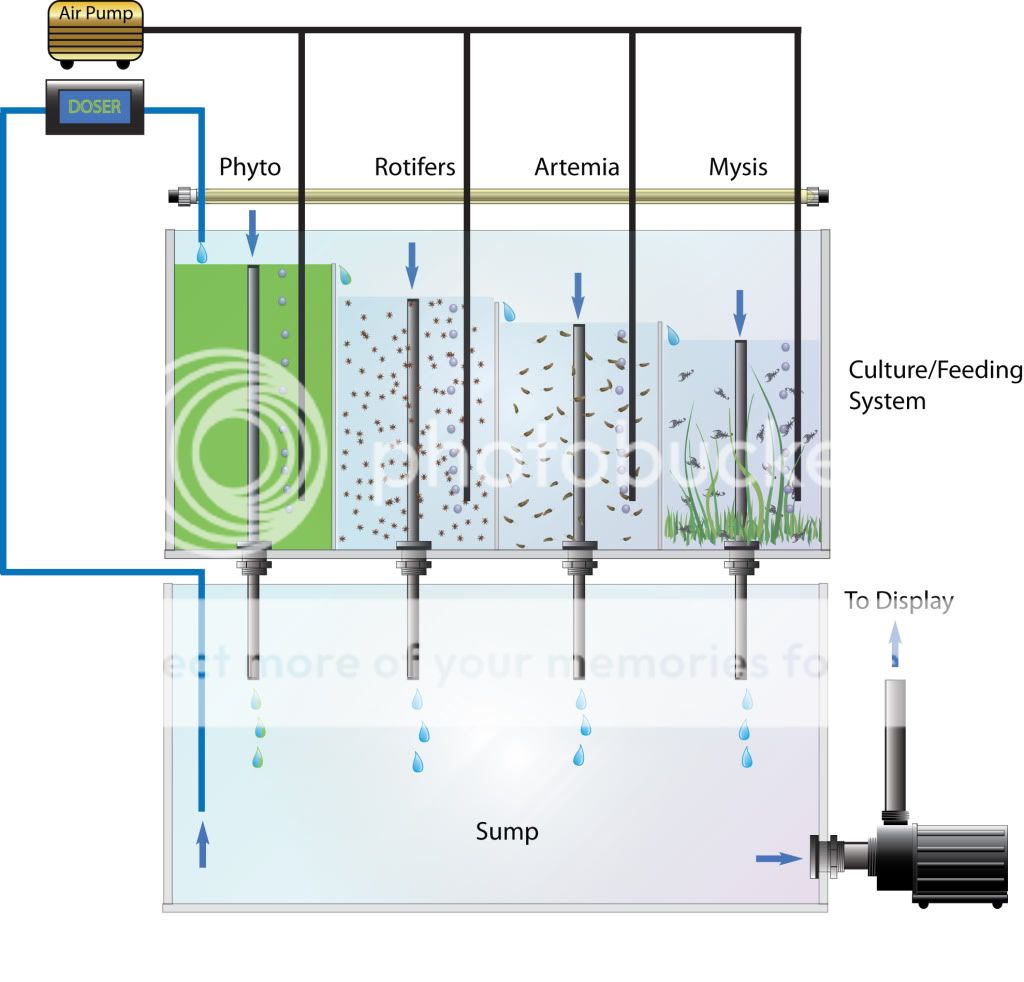
And secondly (Good God I'm long winded; my apologies) I'm extremely curious about your plans for a plankton reactor system. I've always loved this idea, but the implementation seems pretty challenging. Phyto cultures like to crash at the drop of a hat (or a drop of water containing a predator), trophic considerations suggest you need much larger cultures of lower trophic levels, etc. Plus you need to consider that phyto needs those pesky mineralized nutrients to grow and if you grow them in happy phyto media and drip that directly into your tank you're essentially feeding the nuisance algae directly. I remember phyto reactors being discussed a lot a few years back and would be really curious to hear people's experiences with them since.
Batch culturing may have some advantages for autotrophic organisms in that they'll eventually soak up all the nutrients they can before being added to the aquarium, whereas in a chemostat-style continuous culture your output is going to be practically devoid of whatever the growth-limiting nutrient is but with surplus quantities of all other nutrients. You'll also be selecting for different characteristics in the two environments -- cells in the batch culture will tend to evolve faster rates of growth, while those in the chemostat will enhance their ability to suck out ever more quantities of the limiting nutrient out of the media. I'm not sure how practically relevant these considerations are to your project.
My feeling is that a system that combined several large enclosed (to keep out predators) periodic batch phyto culture system with controllable peristaltic delivery to your predator cascade might make for an interesting and tunable experiment. I have a hunch that your predator growth rates might not end up matching very evenly -- you'd be likely to have trouble maintaining acceptable densities of, say, mysids given the rate at which you were able to maintain an acceptable density of rotifers, for instance -- but it would be easy enough convert this whole thing to a more ad-hoc batch culture as necessary. It might be worth doing some rough estimates of growth rates and such for sizing the various compartments.
Anyway, I find this all fascinating, and thanks again for sharing it with all of us!
Cheers,
-Jon