Ted_C
Active member
Just sharing my expereience and recording my observations - not looking for advice (yet).
Lost my Pink margin fairy wrasse and my tomini tang in my 29 gallon biocube quarantine last night. Same ole story - they fed fine last night and this morning both were gone (they were the only inhabitants in the 29 gallon). I noticed the tomini was breathing fast last night around lights out but didn't pay it alot of mind since I have no expereience with tangs and whether it was normal behavoir or a sign of distress.
The biocube was previosly used to quarantine a bellus angel and 4 neon goby. Prazipro has been added 3 times in total to the tank (over the course of two months) and copper was added once for the last quarantine. In between that time and the addition of the new fish - I ran carbon in the filter basket for a week.
Some general parameters of the biocube: 29 gallon, runing as many ceramic biomedia balls in the refugium area as I can fit. Been running since March I beleive. PVC pipe is used in the display for hiding. I have a spectrapure autotopoff. I control and monitor everything with a neptune apex lite, one mp10 running between 80% to 85% power with night mode running @ 40%, one ecotech radion for lighting. I run the biocube without the glass cover to maximize oxygen exchange. The return to the display has one of those whirlybird flow diverters so that the surface is agitated every time it spins around.
Some general observations of the biocube:
I noticed when the bellus was in the tank, she loved to gulp air. I thought she might just be playing but it may also mean I have a dissolved oxygen problem in the tank.
The biocube has always grown algae on the glass so I do have nitrates / nitrates. I didn't really test it though. I no longer own an ammonia test kit. However, I like to think I could detect ammonia just through the smell of the tank. Our noses can detect scents much more accutely than a test kit. A test kit would only quantify it for us.
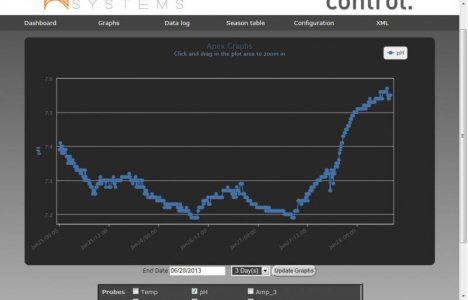
After adding the ecotech radion light over 2 months ago - the temp is stable at 78 degrees. The pH used to be stable - fluctating between 7.8 and 8.1 throughout the day (see the attached graph).
Timeline:
6/15: Bought the fish from Faois. Acclimated via drip for an hour and introduced them.
6/15 - 6/22
Fed them the entire week - small shot of brine in the morning (maybe 10-15 total brine in the tank - probably less than that), 1 small autofeed of pellets/Flake around noon, 1 small shot of mysis in the evening (again no more than 10-15 mysis - again - probably less than that). I also rubber banded some nori to a rock for the tang to munch on.
During this time too - I noticed how big the poops were the tomini was taking. holy cow! it was like a quarter inch in diameter and about an inch long. He musta been proud haha.
6/22:
between 6/23 and 6/24 when I would replace the nori on the rock, I noticed the rock did smell of ammonia. I think this may not be the best idea and should probably stick to feeding nori on a clip or screen instead to allow water flow to penetrate it.
6/24: after re-aquascaping my display to support the addition of a piece of rock from the sump - I took some of the macro algae from that rock and put it on a clip in the quarantine. It may have contained pods and a few Strombus grazers. but it was a small piece.
6/25 Business as usual
6/26 morning - they were dead.
So my possible causes:
a severe drop in dissolved oxygen
An ammonia build up that I didn't detect
The death of one of the fish (especially the big tang) may have spiked the ammonia - causing the other fish to kick the bucket.
I did replace the filter that I have had running in the biocube for these past 4 months. I beleive this filter was covered in the bacteria that was keeping my tank stable. When i removed it, I lost the fish. The ceramic biomedia balls also helped with the stability of the tank but in a lesser degree. I think the tang might have been too much to handle for the 29 gallon biocube.
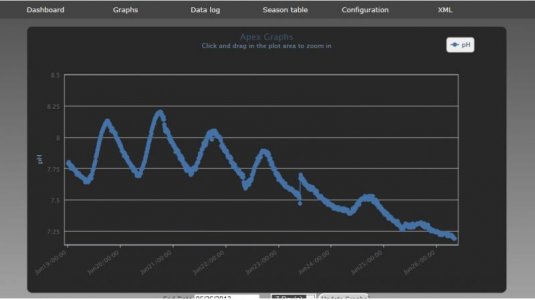
As you can see from the below pH measurements over the last 7 days - I had a problem I didn't know about. pH is currently 7.1 - much too low. Whether this is from Ammonia / Nitric Acid / low dissolved oxygen - this is what caused the fish death.
Lost my Pink margin fairy wrasse and my tomini tang in my 29 gallon biocube quarantine last night. Same ole story - they fed fine last night and this morning both were gone (they were the only inhabitants in the 29 gallon). I noticed the tomini was breathing fast last night around lights out but didn't pay it alot of mind since I have no expereience with tangs and whether it was normal behavoir or a sign of distress.
The biocube was previosly used to quarantine a bellus angel and 4 neon goby. Prazipro has been added 3 times in total to the tank (over the course of two months) and copper was added once for the last quarantine. In between that time and the addition of the new fish - I ran carbon in the filter basket for a week.
Some general parameters of the biocube: 29 gallon, runing as many ceramic biomedia balls in the refugium area as I can fit. Been running since March I beleive. PVC pipe is used in the display for hiding. I have a spectrapure autotopoff. I control and monitor everything with a neptune apex lite, one mp10 running between 80% to 85% power with night mode running @ 40%, one ecotech radion for lighting. I run the biocube without the glass cover to maximize oxygen exchange. The return to the display has one of those whirlybird flow diverters so that the surface is agitated every time it spins around.
Some general observations of the biocube:
I noticed when the bellus was in the tank, she loved to gulp air. I thought she might just be playing but it may also mean I have a dissolved oxygen problem in the tank.
The biocube has always grown algae on the glass so I do have nitrates / nitrates. I didn't really test it though. I no longer own an ammonia test kit. However, I like to think I could detect ammonia just through the smell of the tank. Our noses can detect scents much more accutely than a test kit. A test kit would only quantify it for us.
After adding the ecotech radion light over 2 months ago - the temp is stable at 78 degrees. The pH used to be stable - fluctating between 7.8 and 8.1 throughout the day (see the attached graph).
Timeline:
6/15: Bought the fish from Faois. Acclimated via drip for an hour and introduced them.
6/15 - 6/22
Fed them the entire week - small shot of brine in the morning (maybe 10-15 total brine in the tank - probably less than that), 1 small autofeed of pellets/Flake around noon, 1 small shot of mysis in the evening (again no more than 10-15 mysis - again - probably less than that). I also rubber banded some nori to a rock for the tang to munch on.
During this time too - I noticed how big the poops were the tomini was taking. holy cow! it was like a quarter inch in diameter and about an inch long. He musta been proud haha.
6/22:
- 5 gallon water change (with Red Sea Coral pro).
- Replaced the filter in the media basket - this may have been my downfall.
- Removed the carbon from the media basket
- Dosed 7 ml of Prazi Pro
between 6/23 and 6/24 when I would replace the nori on the rock, I noticed the rock did smell of ammonia. I think this may not be the best idea and should probably stick to feeding nori on a clip or screen instead to allow water flow to penetrate it.
6/24: after re-aquascaping my display to support the addition of a piece of rock from the sump - I took some of the macro algae from that rock and put it on a clip in the quarantine. It may have contained pods and a few Strombus grazers. but it was a small piece.
6/25 Business as usual
6/26 morning - they were dead.
So my possible causes:
a severe drop in dissolved oxygen
An ammonia build up that I didn't detect
The death of one of the fish (especially the big tang) may have spiked the ammonia - causing the other fish to kick the bucket.
I did replace the filter that I have had running in the biocube for these past 4 months. I beleive this filter was covered in the bacteria that was keeping my tank stable. When i removed it, I lost the fish. The ceramic biomedia balls also helped with the stability of the tank but in a lesser degree. I think the tang might have been too much to handle for the 29 gallon biocube.
As you can see from the below pH measurements over the last 7 days - I had a problem I didn't know about. pH is currently 7.1 - much too low. Whether this is from Ammonia / Nitric Acid / low dissolved oxygen - this is what caused the fish death.