Recently I asked a question about digital image colorimetry for reading colorimetric test results. (http://reefcentral.com/forums/showthread.php?t=2566871). Since then I looked more deeply into the subject and now report how digital image colorimetry can be used with the API ammonia test kit to detect levels of total ammonia below 0.1 ppm.
Digital image colorimetry involves taking a picture of the colored test sample and a blank under the same lighting conditions. An app, like Photoshop or ColorMeter, is needed to read the RGB values of the colored and blank solutions. For this test the R or red value is of interest because it is strongly absorbed by blue-green test solution. The deeper the blue-green color, the smaller the R value. The absorbance is calculated and compared to a calibration curve to determine the total ammonia concentration (see photo below). For best results, the solution should be clear and free of particulate matter. For this reason, the API test was modified.
The standard API test gives a very cloudy solution when the test sample is saltwater because the analytical reaction requires a high pH. This precipitate makes detecting low concentrations of ammonia impossible. To prevent the precipitate, about 0.1 mL of solid sodium citrate is dissoved in the 5 mL test sample. In addition only half the amount of each reagent is used and the development time is increased to 10 minutes. This gives a clear, precipitate free test solution. This protocol would make possible the use of a Hanna Checker in place of a camera to detect red light absorption.
The limit of detection, the lowest amount of ammonia this test procedure can detect is estimated to be about 0.05 ppm. Since only three samples were tested at each concentration, many more sample tests are needed to provide an accurate estimate of the limit of detection.
I will be repeating this study with API and Salifert phosphate tests and Salifert nitrate test.
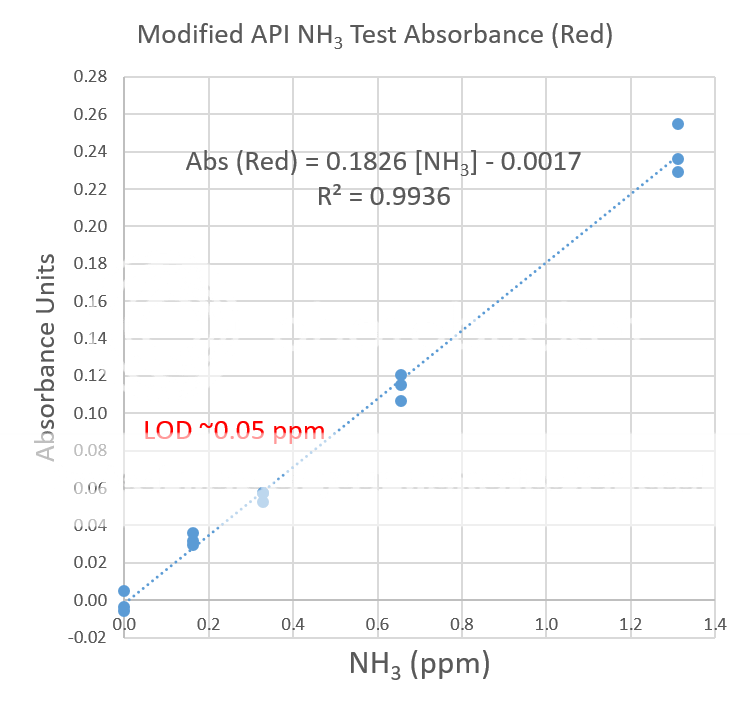
Digital image colorimetry involves taking a picture of the colored test sample and a blank under the same lighting conditions. An app, like Photoshop or ColorMeter, is needed to read the RGB values of the colored and blank solutions. For this test the R or red value is of interest because it is strongly absorbed by blue-green test solution. The deeper the blue-green color, the smaller the R value. The absorbance is calculated and compared to a calibration curve to determine the total ammonia concentration (see photo below). For best results, the solution should be clear and free of particulate matter. For this reason, the API test was modified.
The standard API test gives a very cloudy solution when the test sample is saltwater because the analytical reaction requires a high pH. This precipitate makes detecting low concentrations of ammonia impossible. To prevent the precipitate, about 0.1 mL of solid sodium citrate is dissoved in the 5 mL test sample. In addition only half the amount of each reagent is used and the development time is increased to 10 minutes. This gives a clear, precipitate free test solution. This protocol would make possible the use of a Hanna Checker in place of a camera to detect red light absorption.
The limit of detection, the lowest amount of ammonia this test procedure can detect is estimated to be about 0.05 ppm. Since only three samples were tested at each concentration, many more sample tests are needed to provide an accurate estimate of the limit of detection.
I will be repeating this study with API and Salifert phosphate tests and Salifert nitrate test.

