You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Performing PO4 experiment relating to SPS color/growth
- Thread starter greengeco82
- Start date
bif24701
New member
If that much growth is slow for 15 days then my corals are hibernating.
I know, wish I had that kind of growth!
Sent from my iPhone using Tapatalk
JonezNReef
Member
My all consumption has increased. I can't say by how much. I have an auto doser and it always doses the same amount. Usually my all is about 9 dkh. I checked last week and it was between 7 and 8 dkh. I run 2 x 250w halide with a led supplement along the front of the tank. The frags are in the middle of my tank, so they have a light on each side. There is no shadowing, but the lights are not very intense there. I'll keep up the experiment, since people seem interested.
I find it quite interesting when someone takes the time to experiment around and finds out if something helps, hurts or has no effect on a problem we all run into.
+1 on that! Looks like I might need to change what Im doing so I can get that kind of growth LOLIf that much growth is slow for 15 days then my corals are hibernating.
greengeco82
Reefer
If that much growth is slow for 15 days then my corals are hibernating.
I know, wish I had that kind of growth!
I find it quite interesting when someone takes the time to experiment around and finds out if something helps, hurts or has no effect on a problem we all run into.
+1 on that! Looks like I might need to change what Im doing so I can get that kind of growth LOL
Thanks to all of you. I guess I couldn't see the growth, or just attributed it to a slightly different camera view. Its hard to see growth when you look at your tank 10 times a day.
Phosphate consumption seems to be slowing. I initially was dosing about 12ml of the NeoPhos once a day (sometimes twice). I would get a rise in measurable PO3, then 12 hours later, non detectable again. For the last 7 days I have decreased to 7.5ml once a day only. My tests result in PO4 of 0.0-.16 the several times I tested throughout the week. Since the .16 makes me nervous, i am going to reduce to 5ml/day.
Bagabaga
Member
I'm really interested in this topic because it seems we chase numbers a lot and some tanks aren't stable enough to produce reliable results. I had a short spurt where I noticed my growth, then paeans went haywire from an alk overdose so now I'm recovering again, manual 2 part dosing is hard. Wish I had an auto dosing system.
Not seeing the reaction i wanted. I will probably stop this experiment soon. . .
Since it takes weeks to months for corals to make new fluorescing and chromo proteins I think you should keep running this experiment a while longer. But if you're really trying to get bright colors corals make fluorescing proteins and put them above their zooxanthellae in response to light levels brighter than the zooxantheallae want.
greengeco82
Reefer
But if you're really trying to get bright colors corals make fluorescing proteins and put them above their zooxanthellae in response to light levels brighter than the zooxantheallae want.
Do you care to elaborate? With complete respect, I have no idea what you are referring to.
greengeco82
Reefer
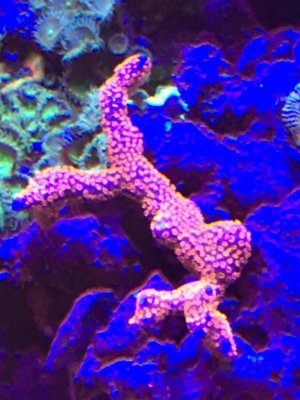
The last coral along with a few others in my tank seems to go through a cycle of burnt tips. Its very obvious in the Day 21 picture. Its been happening 6+ months. Seems to happen about every 10 days. Then recovers nicely, then burns again. Not sure why, but NO3 is between 30 and 40.
Do you care to elaborate? With complete respect, I have no idea what you are referring to.
First, you're seeing if you can make your corals brighter. It takes corals awhile to make new or additional fluorescing proteins so they look brighter. Instead of running your experiment for just a few weeks I think you should consider running it for several months.
Maybe this will help clarify why corals make thier fluorescing protiens, there are at least 4 primary purposes.
1. Photoprotection When the light is too intense for the zooxanthellae the fluorescing oragnelles are placed above them shading them, reducing the amount of light they recieve. As the light levels increase the coral makes more proteins intensifying the colors.
http://www.nature.com/nature/journal/v408/n6814/full/408850a0.html
https://researchonline.jcu.edu.au/4244/1/4244_Salih_et_al_2006.pdf
2. Photoenhancement. If the light is not bright enough for the zooxantheallae to work at optimum photosynthetic effeceincy the fluorescing organelles are located behind them with respect to the light source so they receive increased light levels.
http://www.nature.com/nature/journal/v408/n6814/full/408850a0.html
https://researchonline.jcu.edu.au/4244/1/4244_Salih_et_al_2006.pdf
(With ULNS methods starving the corals makes them look brighter because the zooxanthellae numbers are restricted reducing thier brown componet to the corals coloration.)
3. As antioxidants. The fluorescing proteins are used to neutralize the free radicals caused by photosythesis.
https://www.researchgate.net/publication/26873319_Coral_Fluorescent_Proteins_as_Antioxidants
4. As an Immune response. Fluorescing proteins are used by the corals immune system to deal with parasites.
http://www.jstor.org/stable/25470724?seq=1#page_scan_tab_contents
greengeco82
Reefer
Thanks Tim Fish for the clarification. This is new info for me. cant change the intensity, but I can increase the duration of lighting. I have my halides running for 7 hours. I could try bumping that to 8 or 9 hours.
greengeco82
Reefer
Following. Please keep it up.
Thanks for the encouragement.
Sent from my SAMSUNG-SGH-I337 using Tapatalk
I agree! Please do keep up the experiment!
Thanks, I will.
Optionman
New member
bif24701
New member
First, you're seeing if you can make your corals brighter. It takes corals awhile to make new or additional fluorescing proteins so they look brighter. Instead of running your experiment for just a few weeks I think you should consider running it for several months.
Maybe this will help clarify why corals make thier fluorescing protiens, there are at least 4 primary purposes.
1. Photoprotection When the light is too intense for the zooxanthellae the fluorescing oragnelles are placed above them shading them, reducing the amount of light they recieve. As the light levels increase the coral makes more proteins intensifying the colors.
http://www.nature.com/nature/journal/v408/n6814/full/408850a0.html
https://researchonline.jcu.edu.au/4244/1/4244_Salih_et_al_2006.pdf
2. Photoenhancement. If the light is not bright enough for the zooxantheallae to work at optimum photosynthetic effeceincy the fluorescing organelles are located behind them with respect to the light source so they receive increased light levels.
http://www.nature.com/nature/journal/v408/n6814/full/408850a0.html
https://researchonline.jcu.edu.au/4244/1/4244_Salih_et_al_2006.pdf
(With ULNS methods starving the corals makes them look brighter because the zooxanthellae numbers are restricted reducing thier brown componet to the corals coloration.)
3. As antioxidants. The fluorescing proteins are used to neutralize the free radicals caused by photosythesis.
https://www.researchgate.net/publication/26873319_Coral_Fluorescent_Proteins_as_Antioxidants
4. As an Immune response. Fluorescing proteins are used by the corals immune system to deal with parasites.
http://www.jstor.org/stable/25470724?seq=1#page_scan_tab_contents
Now my question is what method would produce the brighter colors? Protector enhancement?
Sent from my iPhone using Tapatalk
Yes. In theory, a combination of high light, with generous uv component (corals really wanna protect symbiont from that) and low nutrients to keep the symbiont from growing much will give you lots of fluorescent proteins, and not much brown symbiont to dull the color.Now my question is what method would produce the brighter colors? Protector enhancement?
Sent from my iPhone using Tapatalk
But it's debatable whether that is actually good for the coral host, even in theory - set aside the practical difficulty of balancing the tightrope.
Sent from my SAMSUNG-SGH-I337 using Tapatalk
greengeco82
Reefer
Day 31
 Day 1 by [url=https://flic.kr/p/UeJ929]
Day 1 by [url=https://flic.kr/p/UeJ929] Day 31 by
Day 31 by
[url=https://flic.kr/p/Taf2gZ] Day 1 by [url=https://flic.kr/p/UimX1D]
Day 1 by [url=https://flic.kr/p/UimX1D] Day 31 by
Day 31 by
[url=https://flic.kr/p/SLHeVQ] Day 1 by [url=https://flic.kr/p/T4KkaV]
Day 1 by [url=https://flic.kr/p/T4KkaV] Day 31 by
Day 31 by
Since the last update, I increased lighting from 6 hours to 8 hours a day. When I had low nutrients in the past, everything would bleach out. Now corals seem to be taking the increased lighting with no ill effects.
 Day 1 by [url=https://flic.kr/p/UeJ929]
Day 1 by [url=https://flic.kr/p/UeJ929] Day 31 by
Day 31 by [url=https://flic.kr/p/Taf2gZ]
 Day 1 by [url=https://flic.kr/p/UimX1D]
Day 1 by [url=https://flic.kr/p/UimX1D] Day 31 by
Day 31 by [url=https://flic.kr/p/SLHeVQ]
 Day 1 by [url=https://flic.kr/p/T4KkaV]
Day 1 by [url=https://flic.kr/p/T4KkaV] Day 31 by
Day 31 by Since the last update, I increased lighting from 6 hours to 8 hours a day. When I had low nutrients in the past, everything would bleach out. Now corals seem to be taking the increased lighting with no ill effects.
Similar threads
- Replies
- 1
- Views
- 724
- Replies
- 4
- Views
- 2K
- Replies
- 7
- Views
- 1K
- Replies
- 5
- Views
- 946