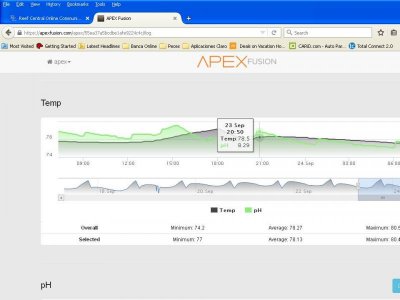
There are many questions from folks regarding keeping pH up in a reeftank. Too many use commercial buffers designed to increase alkalinty( and often marketed as pH panaceas) to pursue and ideal pH without success in managing pH or alkalinity as the outcome.
So , I'll offer a little information on what works and doesn't for managing pH in a reef tank in my experience along with some of the chemistry involved.
This is from a post of mine in a recent thread on the chemistry forum. The focus is on CO2( carbon dioxide) because the CO2 level in the water drives pH in the tank:
CO2 in the ocean stays in sync with atmospheric CO2 ,a trace gas in the atmosphere currently at 390ppm by volume. Obviously the gas exchange rate in the sea is much greater than in droplet of water in a reef tank packed with life. Just think about the surface areas where the water interfaces with the air involved and that 's very clear.
Where does the CO2 come from in a reef tank?
Much of it comes from the surrounding air . A tank with adequate surface area will equillibrate with the air more quickly for better or worse . If the room air is high in CO2,ie, higher than 390ppm ,like when lots of folks are breathing in a closed room for example ;then the CO2 in the tank will rise via gas exchange ,accordingly.
The CO2 also comes from biological activity as organisms from animals to bacteria respire it ; just as you and I breathe it out when we exhale. Given the concentration of life in an aquarium this biological activity can outpace the gas exchange .
On other hand some organisms take it in like corals ,plants and algae that use it, water and the energy from light to meet some of their needs for organic carbon( sugars) via photosynthesis.
It is not uncommon for reef tanks with low gas exchange and heavy photosynthetic activity to have chronically high ph, especially during the lights on period of the day.
Much of the The CO2 in a tank comes from the air around it .The rate of exchange is dependent on the amount of surface interface with the air. Surface agitation increases the volume of surface area . Keeping the surrounding air "fresh" helps to keep it near 390ppm.
The amount of CO2 in the tank is always changing as it comes in and leaves via gas exchange at the surface, is taken up by photosynthesis or in some cases by chemo autotrophic organisms .It is obviously highly variable from tank to tank.
How does CO2 affect pH?
It hydrolizes in the water instantly: CO2+H2O then CH2O3(carbonic acid).
Notice 2 H protons are now in the carbonic acid at the expense of one water molecule. (Actually in salt water it's less often carbonic acid but just free H that occurs but that nuance confuses things ,so will pass it by for now;it's still 2 new H protons ).
pH is a measure of H. So the additional H protons lower the pH.
Since alkalinity is a measure of the capacity of ions (like carbonate, bicarbonate, orthophospahte ,borate and a few others) to take up H ,then more alkalinity will raise pH, as more H is bound to these ions and neutralized ;wont it?
Well, no; not for long because more CO2 from the air will just add more H. Thus, leaving more alkalinity and virtually the same pH except in more acidic tanks.
Note:
(96.5% of the alkalinity in seawater is carbonate alkalinity ( CO3 and HCO3) This is what corals and other calcareous organisms use to make calcium carbonate skeletal mass. It is the carbonate alkalinity reef keepers are concerned about .
Total alkalinity is the measure we use as a surrogate measure for carbonate alkalinity.
Here is what carbonate alkalinity looks like in natural seawater:
Carbonate( CO3) ,approximately 20ppm in seawater.
Bicarbonate(HCO3),approximately 110ppm in seawater.
Carbonic acid(H2CO3) roughly 1% of the total of the three.
These two species of carbonate alkalinity shift instantaneously in response to the amount of H in the water; ie, they are pH dependent :
CO3(carbonate) <---> HCO3( bicarbonate) <---->H2CO3 (carbonic
acid)
Note; CO3 has 2 parking spots for H to neutralize H2CO3(carbonic acid) while HCO3( bicarbonate) has only one. Thus , one unit ofCO3 is two units of alk; while, one unit of HCO3 is only one unit of alk .
More H moves the mix toward higher levels of HCO3 nd H2CO3 ; less H( higher ph) moves the speciation towards more CO3.
So as CO2 brings in more H it reduces alkalinity :right?
No,because it also adds O from the water molecule it took to make CO2+H2O=H2 CO3(carbonic acid).
The carbonic acid gives one H proton to join a CO3 ion ( 2 units of alkalinity) making it an HCO3 ion ( 1 unit of alkalinity) .
After the carbonic acid gives that H proton to CO3 ,it is now HCO3(1 unit of alkalinity).
H2CO3 +C03 -----> HCO3 ,HCO3
So we now have 2 HCO3 ions ( each one unit of alkalinity for a total of 2 alkalinity units ; we gave up one CO3 ion which had 2 units of alkalinity. The alkalinity is unchanged by the CO2; not so the pH as there are now 2 H protons we didn't have before the CO2 entered the water. Thus you can have low pH and high alkalinity.
Dosing alk to control pH is a bad strategy as stable alk is important and the practice won't do much for Ph for very long.
CO2 in the water lowers pH or conversely low CO2 in the water raises pH.
Methods to manage it include:
Fresh room air .
Enhanced open surface areas and increased surface agitation.
Outside air runs to the skimmer air intake
Opposite photo period refugia and/ or in some multiple tank systems different photoperiods for different tanks.
Dosing calcium hydroxide(kalk) in lieu of two part supplements or calcium and alkalinity since it uses CO2 to make CO3.
CO2 scrubbers attached to skimmer air intakes.
So , I'll offer a little information on what works and doesn't for managing pH in a reef tank in my experience along with some of the chemistry involved.
This is from a post of mine in a recent thread on the chemistry forum. The focus is on CO2( carbon dioxide) because the CO2 level in the water drives pH in the tank:
CO2 in the ocean stays in sync with atmospheric CO2 ,a trace gas in the atmosphere currently at 390ppm by volume. Obviously the gas exchange rate in the sea is much greater than in droplet of water in a reef tank packed with life. Just think about the surface areas where the water interfaces with the air involved and that 's very clear.
Where does the CO2 come from in a reef tank?
Much of it comes from the surrounding air . A tank with adequate surface area will equillibrate with the air more quickly for better or worse . If the room air is high in CO2,ie, higher than 390ppm ,like when lots of folks are breathing in a closed room for example ;then the CO2 in the tank will rise via gas exchange ,accordingly.
The CO2 also comes from biological activity as organisms from animals to bacteria respire it ; just as you and I breathe it out when we exhale. Given the concentration of life in an aquarium this biological activity can outpace the gas exchange .
On other hand some organisms take it in like corals ,plants and algae that use it, water and the energy from light to meet some of their needs for organic carbon( sugars) via photosynthesis.
It is not uncommon for reef tanks with low gas exchange and heavy photosynthetic activity to have chronically high ph, especially during the lights on period of the day.
Much of the The CO2 in a tank comes from the air around it .The rate of exchange is dependent on the amount of surface interface with the air. Surface agitation increases the volume of surface area . Keeping the surrounding air "fresh" helps to keep it near 390ppm.
The amount of CO2 in the tank is always changing as it comes in and leaves via gas exchange at the surface, is taken up by photosynthesis or in some cases by chemo autotrophic organisms .It is obviously highly variable from tank to tank.
How does CO2 affect pH?
It hydrolizes in the water instantly: CO2+H2O then CH2O3(carbonic acid).
Notice 2 H protons are now in the carbonic acid at the expense of one water molecule. (Actually in salt water it's less often carbonic acid but just free H that occurs but that nuance confuses things ,so will pass it by for now;it's still 2 new H protons ).
pH is a measure of H. So the additional H protons lower the pH.
Since alkalinity is a measure of the capacity of ions (like carbonate, bicarbonate, orthophospahte ,borate and a few others) to take up H ,then more alkalinity will raise pH, as more H is bound to these ions and neutralized ;wont it?
Well, no; not for long because more CO2 from the air will just add more H. Thus, leaving more alkalinity and virtually the same pH except in more acidic tanks.
Note:
(96.5% of the alkalinity in seawater is carbonate alkalinity ( CO3 and HCO3) This is what corals and other calcareous organisms use to make calcium carbonate skeletal mass. It is the carbonate alkalinity reef keepers are concerned about .
Total alkalinity is the measure we use as a surrogate measure for carbonate alkalinity.
Here is what carbonate alkalinity looks like in natural seawater:
Carbonate( CO3) ,approximately 20ppm in seawater.
Bicarbonate(HCO3),approximately 110ppm in seawater.
Carbonic acid(H2CO3) roughly 1% of the total of the three.
These two species of carbonate alkalinity shift instantaneously in response to the amount of H in the water; ie, they are pH dependent :
CO3(carbonate) <---> HCO3( bicarbonate) <---->H2CO3 (carbonic
acid)
Note; CO3 has 2 parking spots for H to neutralize H2CO3(carbonic acid) while HCO3( bicarbonate) has only one. Thus , one unit ofCO3 is two units of alk; while, one unit of HCO3 is only one unit of alk .
More H moves the mix toward higher levels of HCO3 nd H2CO3 ; less H( higher ph) moves the speciation towards more CO3.
So as CO2 brings in more H it reduces alkalinity :right?
No,because it also adds O from the water molecule it took to make CO2+H2O=H2 CO3(carbonic acid).
The carbonic acid gives one H proton to join a CO3 ion ( 2 units of alkalinity) making it an HCO3 ion ( 1 unit of alkalinity) .
After the carbonic acid gives that H proton to CO3 ,it is now HCO3(1 unit of alkalinity).
H2CO3 +C03 -----> HCO3 ,HCO3
So we now have 2 HCO3 ions ( each one unit of alkalinity for a total of 2 alkalinity units ; we gave up one CO3 ion which had 2 units of alkalinity. The alkalinity is unchanged by the CO2; not so the pH as there are now 2 H protons we didn't have before the CO2 entered the water. Thus you can have low pH and high alkalinity.
Dosing alk to control pH is a bad strategy as stable alk is important and the practice won't do much for Ph for very long.
CO2 in the water lowers pH or conversely low CO2 in the water raises pH.
Methods to manage it include:
Fresh room air .
Enhanced open surface areas and increased surface agitation.
Outside air runs to the skimmer air intake
Opposite photo period refugia and/ or in some multiple tank systems different photoperiods for different tanks.
Dosing calcium hydroxide(kalk) in lieu of two part supplements or calcium and alkalinity since it uses CO2 to make CO3.
CO2 scrubbers attached to skimmer air intakes.
Last edited: