No, their shells are not soluble at the pressure levels in our tanks. They will dissolve deeper in the ocean.when they die, they release the silicates back? It's a closed system unless you extract them with skimming or water changes.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Dinoflagellates.
- Thread starter DNA
- Start date
DNA
New member
Over the last 3 weeks or so I've done around 10 siphons of the sandbed.
One would expect to get less and less gunk out when it's done this frequently, but that is not the case.
The amounts are simply amazing every time.
I took a closer look at the lightest particles and found one type of special interest.
It has the color and similar shape to my ostis but is more uniform, larger and they don't move.
I kept wondering if these are cysts so I melted all the calcium based particles off just to get that possibility out of the way.
It surely looks organic.
Being in their millions in the sand that is close to solely inhabited with dinos it's tempting to assume they are cysts.
I calculated the average size of these particles to 44 microns, examined the photographs further and compared to material found on the web.
The size is good for both cysts and cells and since they come in various sizes it's not a dead giveaway.
The shape is a close, but not a perfect match to cysts so I think what I have are regular dinos that have lost their cell wall. (theca)
If that is the case all dead dinos do that since there is no other form found in the sample of thousands.
mikeatjac
Active member
After reading this thread twice I got no answers.
So over the course of 3 weeks I;
Weekly 100% water changes with IO (no organics)
Loaded up my carbon reactor.
Double the gfo I was running.
Raised my ph to 11
Ran my tank with blue lights only
Changed my felt socks 3 times a day.
Removed all sand.
Today I can find not a trace. Corals look good even they were exposed to the air for 30 minutes. Dinos are probably still there so I will continue this process for another two weeks.
So over the course of 3 weeks I;
Weekly 100% water changes with IO (no organics)
Loaded up my carbon reactor.
Double the gfo I was running.
Raised my ph to 11
Ran my tank with blue lights only
Changed my felt socks 3 times a day.
Removed all sand.
Today I can find not a trace. Corals look good even they were exposed to the air for 30 minutes. Dinos are probably still there so I will continue this process for another two weeks.
robertifly
Premium Member
Mike I applaud you for taking affirmative action and I certainly hope continued (Dino Free) success. I know we are all as frustrated as you are, seems like someone should have found a tried and true method to eliminate these things by now. I only hope we can all keep trying and sharing as you have, maybe we'll have an answer soon.
jason2459
Well-known member
I've definitely confirmed what I already knew and that dinos (not just the good kinds) still live in my tank. They just don't flourish or at least show that they do in my display tank.
And I have doubts that any tank that has shown dino issues in the past are ever truly pest dino free.
And I have doubts that any tank that has shown dino issues in the past are ever truly pest dino free.
karimwassef
Active member
I've definitely confirmed what I already knew and that dinos (not just the good kinds) still live in my tank. They just don't flourish or at least show that they do in my display tank.
And I have doubts that any tank that has shown dino issues in the past are ever truly pest dino free.
I doubt any tank is dino or dino cyst free. It's like saying that a tank is bacteria free.
jason2459
Well-known member
I agree but I don't like all encompassing ALL statements.I doubt any tank is dino or dino cyst free. It's like saying that a tank is bacteria free.
DNA
New member
Originally Posted by DNA View Post
Over the last 3 weeks or so I've done around 10 siphons of the sandbed.
One would expect to get less and less gunk out when it's done this frequently, but that is not the case.
The amounts are simply amazing every time.
I took a closer look at the lightest particles and found one type of special interest.
It has the color and similar shape to my ostis but is more uniform, larger and they don't move.
I kept wondering if these are cysts so I melted all the calcium based particles off just to get that possibility out of the way.
It surely looks organic.
Being in their millions in the sand that is close to solely inhabited with dinos it's tempting to assume they are cysts.
I calculated the average size of these particles to 44 microns, examined the photographs further and compared to material found on the web.
The size is good for both cysts and cells and since they come in various sizes it's not a dead giveaway.
The shape is a close, but not a perfect match to cysts so I think what I have are regular dinos that have lost their cell wall. (theca)
If that is the case all dead dinos do that since there is no other form found in the sample of thousands.
Detritus collects in my overflow box.
Today I took a sample from the bottom of it and at least 95% are these particles.
Of the rest I assume 4% to be the same particles broken up and 1% unknown.
A quick conclusion could be that 99% of the drifting ditritus in my tank is dino related.
---
Now turn on your brains and think for a while. I see this as another milestone.
.
DNA
New member
A day of discovery.
This is the first time I see on print why the dinos and cyano like to hang out together.
+
---
Most dinoflagellates are encased in plates of armor.
Dinoflagellates are surrounded by a complex covering called the amphiesma, which consists of outer and inner continuous membranes, and between which lie a series of flattened vesicles. In armored forms, these vesicles contain the thecal plates, cellulose plates that are the "armor". This armor may be lacking (the cells are "naked"), and some species shed their theca under certain environmental conditions.
Armored dinoflagellates have two major plate regions composed of two to 100 individual plates. The edges of the plates overlap, sliding apart as the cell increases in size and allowing the cell to expand. The plates come in many varied shapes, from spherical forms like Peridinium to elongate horn-like forms such as Ceratium. In addition, some species have ridges or crests -- especially members of the Dinophysiales, such as the one shown at right. In some, the crests may be hollow and house cyanobacteria which provide fixed nitrogen to the host. This is most common in nitrogen-poor waters.
Source: http://www.ucmp.berkeley.edu/protista/dinoflagmm.html
This is the first time I see on print why the dinos and cyano like to hang out together.
+
---
Most dinoflagellates are encased in plates of armor.
Dinoflagellates are surrounded by a complex covering called the amphiesma, which consists of outer and inner continuous membranes, and between which lie a series of flattened vesicles. In armored forms, these vesicles contain the thecal plates, cellulose plates that are the "armor". This armor may be lacking (the cells are "naked"), and some species shed their theca under certain environmental conditions.
Armored dinoflagellates have two major plate regions composed of two to 100 individual plates. The edges of the plates overlap, sliding apart as the cell increases in size and allowing the cell to expand. The plates come in many varied shapes, from spherical forms like Peridinium to elongate horn-like forms such as Ceratium. In addition, some species have ridges or crests -- especially members of the Dinophysiales, such as the one shown at right. In some, the crests may be hollow and house cyanobacteria which provide fixed nitrogen to the host. This is most common in nitrogen-poor waters.
Source: http://www.ucmp.berkeley.edu/protista/dinoflagmm.html
In addition, some species have ridges or crests -- especially members of the Dinophysiales, such as the one shown at right. In some, the crests may be hollow and house cyanobacteria which provide fixed nitrogen to the host. This is most common in nitrogen-poor waters. [/I]
Source: http://www.ucmp.berkeley.edu/protista/dinoflagmm.html
Here's another fun one that discusses what the cyano gets out of the deal.
They found dinos - some in very deep water - that hosted cyano in the armor and sometimes within the cell itself.
"We propose that heterotrophic dinoflagellate hosts may provide the cyanobacterial symbionts with the anaerobic microenvironment necessary for efficient N fixation. Thus, these self-supporting consortia increase in numbers during the long period of stratification and nitrogen limitation in the oligotrophic subtropical waters of the Gulf of Aqaba."
I wonder if there's anything you could do to determine whether these are 'asleep' in their cysts or dead. Didn't you get reinvigorated blooms by adding Ca/Alk?Detritus collects in my overflow box.
Today I took a sample from the bottom of it and at least 95% are these particles.
Of the rest I assume 4% to be the same particles broken up and 1% unknown.
Could you pull these out into a container and simulate adding Ca/Alk to see if they 'wake up'?
I've definitely confirmed what I already knew and that dinos (not just the good kinds) still live in my tank. They just don't flourish or at least show that they do in my display tank.
And I have doubts that any tank that has shown dino issues in the past are ever truly pest dino free.
Agreed 100% and I suspect that most people with a dino outbreak have multiple species present, and I'll bet treatments that work in some tanks but "fail" in others are "failing" in that they reduce populations of some kinds, while another species increases.
It likely explains a lot of failures of "one method at a time" approaches.
karimwassef
Active member
I still think that nitrogen fixation was a key to this.. at least in my tank.
Ok so although no visible signs of dino's for 10 days there are still some there when I take samples from filter sock and anything I siphon out. Maybe four or five here and there. However, thinking I had another bloom of them with brown areas developing and odd spots of bubbles I scraped some of the brown off and seems I now have a massive outbreak of diatoms! So any suggestions, should I leave them? Seems like some are feeding on dead dino's as well. Not sure what to do now?
Attachments
DNA
New member
Anyone recognizes these particles?
Hardly visible with the naked eye and a bit larger than dinos.
Particles
Sinks from the siphon tube
Hardly visible with the naked eye and a bit larger than dinos.
Particles
Sinks from the siphon tube
Last edited:
Diatoms don't "feed" on dinos. But they will take advantage of nutrients made available by dino deaths.
If you don't want dinos, you need to replace them with something, diatoms have volunteered for the job!
Speaking of diatoms, DNA, scale is a headache, and in a pinch, I've used groups of diatoms as scale bars.
For instance, in joti's post the pigmented part of those diatoms is about 20 microns I think.
Dunno how flawed that method is, but maybe it gets within +-50%
If you don't want dinos, you need to replace them with something, diatoms have volunteered for the job!
Speaking of diatoms, DNA, scale is a headache, and in a pinch, I've used groups of diatoms as scale bars.
For instance, in joti's post the pigmented part of those diatoms is about 20 microns I think.
Dunno how flawed that method is, but maybe it gets within +-50%
Diatoms don't "feed" on dinos. But they will take advantage of nutrients made available by dino deaths.
If you don't want dinos, you need to replace them with something, diatoms have volunteered for the job!
Speaking of diatoms, DNA, scale is a headache, and in a pinch, I've used groups of diatoms as scale bars.
For instance, in joti's post the pigmented part of those diatoms is about 20 microns I think.
Dunno how flawed that method is, but maybe it gets within +-50%
I was going to get a scale slide until I saw how much they cost :O So any suggestions as to how to get rid of the diatoms now?
taricha said:dirty method+light+no skimming+trace elements
Three out of four make sense to me...
34cygni said:Other researchers compared the requirements for the trace elements Fe, Mn, Zn, Cu, Co, and Cd in what I'm guessing were eight common laboratory strains of coccolithophores, diatoms, and dinos -- the three dominant primary producers in the modern oceans -- and found that the dinos had relatively high quotas for all six metals. The diatoms, interestingly, had very low requirements for all six of these micronutrients (though some planktonic diatoms are known to have high requirements for Fe, possibly because they host cyanobacterial endosymbionts) while the cocos had high quotas for Mn, Co, and Cd, but low requirements for iron, zinc, and copper.
Dinos' high requirements for trace elements would explain and confirm these and a number of similar observations over the life of this thread...
06/24/2013, 01:55 PM #1
DNA
Water changes
Dinoflagellates love water changes and not doing them will for sure make the dinos suffer.
08/29/2013, 04:24 PM #19
Squidmotron
5) I agree that water changes -- if anything -- make it worse. They seem to die off more the longer between the water changes. I read a few articles that they like and depend on selenium and iron. Maybe that affects it.
6) Obvious, but do not dose trace elements.
11/19/2013, 10:08 AM #99
bazeball05
stop doing water changes (Dino's are fueled by trace elements)
10/11/2014, 03:26 PM #342
cal_stir
I to am in the midst of a battle with ostreopsis, about six weeks now, I to am convinced that water changes feed it
As for other primary producers, diazotrophic cyano hearts iron because those species need Fe to make nitrogenase, the enzyme they need to fix nitrogen. Green algaes need iron, zinc, and copper; red algaes have higher requirements for Mn, Co, and Cd and lower quotas than greens for Fe, Zn, and Cu.
But if dosing trace is good for dinos, it never looked to me like the flipside of that -- drawing down trace elements -- could be used against them. Even if trace metals are depleted in the water column, the bacterial decay of organic matter in the sand is constantly releasing more (as well as N and P) into the interstitial water, not to mention that mixotrophic dinos can eat the bacteria themselves. To outcompete dinos for trace nutrients would thus require a change of management in the microphytobenthos so the dinos lose control of their source of trace metals.
taricha said:Chaeto and Caulerpa grew well, many many more pods, worms, and general critters took up residence in the sandbed. Dinos directly under the chaeto started to disappear. Maybe it was chemical competition, or predation from the critters living in the chaeto, or reduced light under chaeto, or a combination. Other than directly below chaeto, cyano and dinos continued to grow, even right next to the macros. ...
From what I've seen with the macros in my tank - having looked through the microscope at the sandbed for hours before and after the macroalgae was dropped in. To say there's 10x more benthic fauna of 5x as many species as without the macro likely severely understates the case. And I was going "dirty method" before the macros went in. ...
And the locations of dinos/cyano retreat and reappearance says that proximity to algae is a strong factor.
This sounds like the "DDAM + dinos" model I proposed on page 101 in action. That is, the DDAM model describes competition between corals and algae, ecosystem engineers that shape the composition and function of the reef ecosystem from the bottom up, starting with the bacteria population, and I proposed that dinos fight for the same goal and on the same terms, using organic carbon (and when necessary, their toxins) to recruit and maintain an army of friendly bacteria much as algae and corals do.
34cygni said:Just as coral-friendly bacteria reinforce conditions amenable to coral and algae-friendly bacteria help algae take over reefs, dino-friendly bacteria want to remake the ecosystem to suit dinos.
But while I thought about the possibility of outcompeting benthic dinos for trace and it seemed unlikely to succeed, I didn't turn it around and think about dinos (which as noted have high trace requirements) and their bacteria farms (which also need a lot of trace) outcompeting the rest of the system for trace elements.
To me, taricha, what your report suggests is that dinos don't like to let nutrients pass up the food chain. I mean, yes, the argument can be made that dinos would naturally want to kill off any potential predators, and by so doing, it happens that they sever the trophic link between the world of single-celled organisms and the macro world of multicellular life... But the counterpoint is that the dinoflagellate holobiont is pretty much a biological desert even after more than 200 million years and several mass extinctions which afforded dinos plenty of opportunities to recruit and build up their team, so it would seem that sharing simply isn't compatible with their way of life (...which may explain why they appear to be declining on a long-term, evolutionary time scale -- dinos, appropriately enough, peaked when dinosaurs ruled the world). Recall that dinos are predators that acquired the ability to photosynthesize, not autotrophs that learned to hunt, so I suspect that in their heart of hearts, they want to be on top of the food chain. To that end, dinos try to sequester important nutrients in the microbial loop -- or, to put it another way, in their bacteria farms: when a dino dies, its decay feeds bacteria, and the bacteria and the nutrients released by the decay process feed the dinos.
Because the microphytobenthos -- the primary producers living in the uppermost couple of millimeters of sand where enough light penetrates to support photosynthesis -- can absorb both nutrients in the water column diffusing into the sand and also nutrients in the interstitial water diffusing up out of the sand, organisms occupying this ecological niche largely regulate the exchange of nutrients between the water and the sediment. As most nutrient cycles can only be closed by anaerobes living in the sand and rock, this puts dinos in the catbird seat. Changing the benthic bacteria population and by so doing, changing the flux of nutrients coming out of the sediments, is fundamental to the fight between corals and algae for dominance in a reef environment: corals want the benthic community to release food as particulate, ideally living, organic carbon and produce net surplus O2 over the course of a day; algae want the benthic environment to be dominated by heterotrophs, in oxygen deficit, and releasing mineralized nutrients.
Dinos live in the ecological sweet spot that corals and algae are trying to manipulate for their own benefit, so naturally dinos manipulate it for theirs. Rather than liberating nutrients for the benefit of macroscopic forms of life, apparently dinos make a point of trying to lock up nitrogen (they can even store surplus N internally as urea) and trace metals in the microbial loop, no doubt because these elements are vital for protein synthesis. Phosphorous, on the other hand, they don't seem so worried about, perhaps because they need surplus P to recruit and farm cyano, and perhaps also because P is primarily consumed during the synthesis of ribosomes and genetic material, which is generally associated with reproduction, which other forms of life won't be doing a lot of if the dinos are hoarding N and trace in the microbial loop.
taricha said:If I had to guess which trace element is responsible, I'd lean towards Iron
Iron is the only micronutrient known to limit primary producers in the wild, so that would be the place the start. If I were a dino, I'd want to lock down the local iron supply because iron-limiting other phototrophs would be the natural "backup system" to turn to if I lost control of the nitrogen supply.
taricha said:It could be any number of other things, there's nothing I've observed that excludes, for instance the Cobalt-B12-Cyano-Dino connection.
Given that dinos have high requirements for trace elements on top of the requirements of the bacteria they depend on, directly or indirectly, to obtain nutrients, it could well be that dinos not only "know" where all the crucial choke points are (like vitamin B-12) but have evolved ways of cornering the market on any micronutrient that potential competitors would need, and can prioritize which element(s) they should invest their energy in monopolizing on the basis of what nutrients are available and which competitors are growing fastest at any given moment.
taricha said:For two days I didn't have time to look at the tank or dose N or P. Just throw in a pinch of food.
Don't overlook the dog that didn't bark. This non-event -- stopping nutrient inputs -- may have factored into the dinos' collapse.
taricha said:So thinking back on it, my dino species count from my tank is 5...
I seriously doubt my tank is all that special. It's not like I scooped up sand samples from 10 different coasts and poured them in my tank. I would be shocked if most plagued tanks don't have at least a couple of different species active at the same time.
Co-occurrence of multiple benthic dinoflagellate species has been observed in the wild, and it's common for pelagic dinoflagellate blooms to go through multiple species successions. As each bloom fades and the dinos decay, another species gets going -- apparently by eating the resulting bacteria bloom.
I previously mentioned that I assumed the presence of multiple species of heterotrophic dinos in hobby systems; ditto for mixos, which is why I added a line to Quiet_Ivy's FAQ about the risk of ending up with tougher, more toxic dinos if you knock an infestation back but don't follow through. And IIRC, Montireef reported getting amphidinium after he knocked back his ostis, so it can potentially go in the other direction, too.
taricha said:Saw some slight brown strings from the exposed "roots" of my caulerpa
And under the scope, dinos!
Interesting that they showed up on the part of the algae that grows underground, as epiphytic typically refers to organisms growing on the "leaves" (...and from what I read, epiphytic dinos will also grow on seagrass, which actually does have leaves). Your caulerpa kept growing prior to the dinos' collapse, IIRC -- it was the exception you reported to several observations suggesting Fe limitation... And you reported spots free of dinos developing under your chaeto, but not the caulerpa...? And, of course, caulerpa is freakishly robust and toxic... I wonder if caulerpa could have "defected" from Team Green Algae and made friends with some of the bacteria in the dino holobiont -- maybe that's how it was able to get iron (or B-12 or whatever) when it was in short supply.
--
DNA said:I just spent some time on google looking at images of dino infested tanks looking for similarities.
What stood out and most seem to have in common is that they are sparsely populated with corals.
Hmm... Maybe I was wrong about this...
34cygni said:jonwright said:So while corals help build up the bacteriolandscape do they really combat the complimentary bacteria for Dino's? It certainly explains why corals suffer. So should you add MORE corals if you have a Dino problem?
The tale about the changing bacteria on the corals taken from the Red Sea, kept in fishbowls, and then put back where they came from was meant in part to address this. Corals will rebuild healthy and diverse bacteria populations all by themselves if they get the chance, so rather than adding new corals, we should be concentrating on saving and building up the ones we have.
That crossed my mind when I was looking at PorkchopExpress' very pretty tank some weeks ago. The DDAM model suggests that adding macro would shift a system towards the algae holobiont, and adding corals should tend to favor the population of coral-friendly bacteria and benthic primary producers at the base of a system's food web.
But while adding more corals might help against entrenched dinos, taricha's approach apparently actually did help, and it looks safer, cheaper, and easier for others to play around with (...excepting the 3 hours of sunlight -- I expect most folks won't be able to implement that, while the minority report will be something along the lines of, "Three hours? Why only three hours?"). If by some miracle it works with your ostis, DNA, you can always shift the tank towards coral dominance afterwards.
DNA said:That led me to think about the natural chemical warfare in reef ecosystems.
A local friend has ostis, but his tank has always looked better than mine and he's got much more coral density than I have had since dinos showed up.
I had a hard time finding the dinos, but they were there. Then he built a connected frag tank and it got covered, in the empty tanks, with dinos right away.
While the chemical warfare is real, the DDAM model says it's mostly about biological warfare -- that is, recruiting bacteria to do your dirty work. It makes sense, if you think about it: bacteria (and archaea) are the real experts on chemical warfare as they've been doing it for 4 billion years and they reproduce and evolve considerably more quickly than multicellular organisms, so standard operating practice is to recruit mercenaries from this population to fight for you. Multicellular organisms have to recruit friendly bacteria just to survive in a world full of bacteria that want to kill and eat us, so it's just a hop, skip, and a jump from there to paying single-celled Hessians to occupy territory and even attack on our behalf.
DNA said:In addition, some species have ridges or crests -- especially members of the Dinophysiales, such as the one shown at right. In some, the crests may be hollow and house cyanobacteria which provide fixed nitrogen to the host. This is most common in nitrogen-poor waters.
taricha said:Here's another fun one that discusses what the cyano gets out of the deal.
They found dinos - some in very deep water - that hosted cyano in the armor and sometimes within the cell itself.
"We propose that heterotrophic dinoflagellate hosts may provide the cyanobacterial symbionts with the anaerobic microenvironment necessary for efficient N fixation. Thus, these self-supporting consortia increase in numbers during the long period of stratification and nitrogen limitation in the oligotrophic subtropical waters of the Gulf of Aqaba."
That may tie into this...
Microbial photosynthesis in coral reef sediments (Heron Reef said:We investigated microphytobenthic photosynthesis at four stations in the coral reef sediments at Heron Reef, Australia. The microphytobenthos was dominated by diatoms, dinoflagellates and cyanobacteria, as indicated by biomarker pigment analysis. Conspicuous algae firmly attached to the sand grains (ca. 100 um in diameter, surrounded by a hard transparent wall) [...note that this sounds a bit like what Quiet_Ivy described as "harder brown circular spots on the glass"] were rich in peridinin, a marker pigment for dinoflagellates, but also showed a high diversity based on cyanobacterial 16S rDNA gene sequence analysis.
If some planktonic dinos find it so worthwhile to have symbiotic cyano around that they evolved little bay windows in their armor to give cyanobacteria a home, it seems perfectly reasonable that some benthic dinos could have evolved a way to build greenhouses for their cyano in the sand. They'd be able to grow a lot more cyano that way. And I've been wondering for months if those spots on Quiet_Ivy's glass were palatial versions of microhabitats that dinos normally build on the surface of sand grains... IIRC, Quiet_Ivy reported dino goo growing from the spots on her glass when things got bad in her tank, so they were clearly important to the ostis.
DNA said:Oh dear.
I just spent a few hours looking at detritus in the wild and found some dinos hanging on the the particles from my previous post and realized I got my sense of scale wrong somewhere.
I still have to find out what they are.
I'm still pulling for forams. Have you been comparing your mystery calcifiers to foraminifera?
--
karimwassef said:It's a philosophy in keeping reefs for me. Everything in my tank replicates a function in nature. I have only two exceptions... Carbon and GFO. Everything else mimics a natural system
Pursuant to that thought, did you keep the cryptic zone you added to your sump? After stumbling across the sponge loop, I'm wondering if you have an opinion on whether or not that was worthwhile on any level.
karimwassef said:who cleans their socks daily?
Physically removing dinos by changing out 10 um filter socks daily (later every other day) was part of cal_stir's routine. He checked his dino population by looking to see what and how many got filtered out when he swapped in a clean one.
05/25/2015, 08:42 PM #1123
cal_stir
I use 10uM filter socks on my drains which I change every 2 days. Looking at the tank I can't tell I have dinos but under the microscope I still see a few in my skimmate and socks but they seem deformed and are weak swimmers, I've started culturing phyto to rebuild the micro fauna and critters that were destroyed by FM algaeX (trying to get rid of bubble algae) which I believe are what keep the dinos in check.
--
bertoni said:karimwassef said:when they die, they release the silicates back? It's a closed system unless you extract them with skimming or water changes.
No, their shells are not soluble at the pressure levels in our tanks. They will dissolve deeper in the ocean.
What's your source on this? You're thinking of CaCO3, I suspect.
In the wild, > 50% of biogenic silica formed in the marine environment dissolves at a depth of < 100 m, and 97% of biogenic silica is recycled in surface waters and on the seafloor, both of which are environments present in comparative abundance in aquaria.
--
Dfee said:Well, I haven't had long brown snotty dinos in about a year. So if they are indeed diatoms, how have they not consumed all their food (silicates) and died out by now?
Do you have dissolved Si in your tap water? Tap water topoffs or a RODI rig with an old/clogged/damaged filter could be putting silicates into your tank.
A lengthy transitional diatom period has been reported by some after knocking down their dinos with the dirty method, so a diatom phase is probably a normal part of a tank's ecology rebuilding itself after the dinopocalypse much as a diatom phase is a normal part of a tank's birth process -- but a year is ridiculous... If you really have been stuck with diatoms for a year and you've ruled out any external source of Si that's keeping them going, you may be able to short-circuit the silicon cycle by introducing aluminum (in the form of powdered basalt or kaolinite) into the substrate to see if you can trigger the formation of aluminosilicate minerals and sequester the Si in the sand. This is very common geochemistry in the wild, but since aluminum is toxic and presumably nobody puts it in their tank, this sink may be inactive in hobby systems.
The interaction between aluminum and the silicon cycle is something I picked up from a book, but the basic idea -- aluminum reduces the release of silicic acid (H4SiO4) from benthic sediments -- is in this abstract. Apparently, replacing about 1 in every 75 atoms of Si in biogenic silica with an atom of Al reduces the silica's solubility by 25%, substantially reducing the efficiency of Si recycling. And while some diatoms are toxic, the edibility of diatoms generally has an inverse relationship to the thickness (and in some species also spikiness) of their silica armor, so lowering the availability of Si by a modest amount may make a big difference to your CUC.
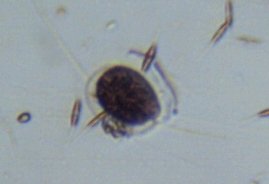
DNA
New member
34cygni:
Good job at connecting the dots.
My particles are not calcium based. I proved that with vinegar.
Given the amounts of them, dino related is all I can think of at the moment.
Here are the calcium based ones I found on my overflow sides recently.
Foraminifera
I measured my calcium at 400 yesterday and that is the highest for a very long time without adding chemicals on top to my calcium reactor and kalkwasser.
The frequent siphoning could have produced this result.
Good job at connecting the dots.
My particles are not calcium based. I proved that with vinegar.
Given the amounts of them, dino related is all I can think of at the moment.
Here are the calcium based ones I found on my overflow sides recently.
Foraminifera
I measured my calcium at 400 yesterday and that is the highest for a very long time without adding chemicals on top to my calcium reactor and kalkwasser.
The frequent siphoning could have produced this result.