You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Fauna Marin Trace unbalanced?
- Thread starter Randy Holmes-Farley
- Start date
Yes, sodium rises too, but at least in two part recipes with a proper recipe, the other cations (calcium, magnesium, potassium, etc) also rise by the same proportion, so when sg is corrected, they return to normal. That's why, for example, with magnesium and calcium, you must add MORE than is taken up in calcification.
I've calculated the rise in sg in several articles, including this one:
How to Select a Calcium and Alkalinity Supplementation Scheme
http://www.advancedaquarist.com/issues/feb2003/chem.htm
from it:
The rise in salinity of these products over time can be very roughly calculated, though there are several reasons why this calculation is only an estimate. For every 1000 meq of alkalinity added in this fashion (and the matching amount of calcium) these products will deliver on the order of 60 grams of other ions to the tank. In a tank with a low calcification demand (defined later to be 18.3 thousand meq of alkalinity per year in a 100 gallon tank (50 meq/day)) this effect will raise the salinity by 3 ppt per year (compared to a normal salinity of S ~35). In a high demand tank (defined later to be 219 thousand meq of alkalinity per year in a 100 gallon tank (600 meq/day)), the salinity will rise by 35 ppt in a year, or approximately doubling the salinity. Consequently, the salinity should be monitored closely in using these types of additives, especially in a tank with high calcification rates.
I've calculated the rise in sg in several articles, including this one:
How to Select a Calcium and Alkalinity Supplementation Scheme
http://www.advancedaquarist.com/issues/feb2003/chem.htm
from it:
The rise in salinity of these products over time can be very roughly calculated, though there are several reasons why this calculation is only an estimate. For every 1000 meq of alkalinity added in this fashion (and the matching amount of calcium) these products will deliver on the order of 60 grams of other ions to the tank. In a tank with a low calcification demand (defined later to be 18.3 thousand meq of alkalinity per year in a 100 gallon tank (50 meq/day)) this effect will raise the salinity by 3 ppt per year (compared to a normal salinity of S ~35). In a high demand tank (defined later to be 219 thousand meq of alkalinity per year in a 100 gallon tank (600 meq/day)), the salinity will rise by 35 ppt in a year, or approximately doubling the salinity. Consequently, the salinity should be monitored closely in using these types of additives, especially in a tank with high calcification rates.
]you say that if I have SG always in check that I could have a big chloride buildup and lack of sulfate?I don't think so,
Why do you doubt it? Earlier in the thread, I clearly showed a graph of how sulfate rises when you only use magnesium sulfate and no magnesium chloride, even with water changes. The same type of effect is true in reverse (i.e., using only magnesium chloride and no magnesium sulfate).
I discuss that in detail here:
An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System
http://reefkeeping.com/issues/2006-02/rhf/index.php
Why do you doubt it? Earlier in the thread, I clearly showed a graph of how sulfate rises when you only use magnesium sulfate and no magnesium chloride, even with water changes. The same type of effect is true in reverse (i.e., using only magnesium chloride and no magnesium sulfate).
I discuss that in detail here:
An Improved Do-it-Yourself Two-Part Calcium and Alkalinity Supplement System
http://reefkeeping.com/issues/2006-02/rhf/index.php
Water changes will help fix the ionic balance, but plain SG adjustments with RO/DI won't. I think Randy has gone into detail on this issue.Yes,I understand that there is imbalance but it can not be a big one over time because of a SG check and waterchange.But the imbalance is excess of chloride and not lack of sulfate.
Jimmy54 you say that if I have SG always in check that I could have a big chloride buildup and lack of sulfate?I don't think so,but If someone could measure this it would be a good proof.Don't forget that we also put large amount of sodium in the tanks.
It's quite simple
Lets enhance this problem just a little bit to get a better picture.
Starting point: A 100 liter tank contains 100* 2.7 g/l = 270 grams of sulfate.
The skimmer has removed 4 liter tank-water in 14 days time which means: 270 - 10.8 = 259.2 grams of sulfate is left or 2.6 g/l
A 10% water-change removes 25.92 grams and adds 27 grams of sulfate which will result in a new concentration of - 2.6 g/l sulfate.
After another 14 days it will be 2,5 g/l and after 6 months this will be 2.1 g/l.
This process will stop pretty much after another 6 months when a concentration of 2 g/l is reached missing 700 mg/l of sulfate
Edit; it will go on till 1,9 g/l is reached ... after one more year
Last edited:
But than again 
For as far the latest ICP analysis are reliable, it seems that FM salt (and TM Pro too) contains a little bit more S than 900 mg/l (or roughly 3*900 = 2700 mg/l SO4)
FM measures 959 mg/l and TM pro reef 986 mg/l.
From this perspective I understand why FM states that sulfate will rise when MgSO4 is used.
Here are the results.
http://zeewaterforum.info/forums/showthread.php?125398-Tritonlab-wateranalyse&p=1087782#post1087782
For as far the latest ICP analysis are reliable, it seems that FM salt (and TM Pro too) contains a little bit more S than 900 mg/l (or roughly 3*900 = 2700 mg/l SO4)
FM measures 959 mg/l and TM pro reef 986 mg/l.
From this perspective I understand why FM states that sulfate will rise when MgSO4 is used.
Here are the results.
http://zeewaterforum.info/forums/showthread.php?125398-Tritonlab-wateranalyse&p=1087782#post1087782
Last edited:
Are you suggesting that the BRS Fauna Balling Lite product should only be used by folks using certain salts that start with excessive sulfate?
How do you know exactly how much sulfate your water change is bringing in??
If you know how much is in the salt mix, either by assuming it matches NSW or you have a measurement, it is an easy calculation.
For example, NSW has about 2700 ppm sulfate. Suppose the tank has 2500 ppm and you do a 10% water change. Then the result is 0.9(2500) + 0.1(2700) = 2520 ppm.
If you know how much is in the salt mix, either by assuming it matches NSW or you have a measurement, it is an easy calculation.
For example, NSW has about 2700 ppm sulfate. Suppose the tank has 2500 ppm and you do a 10% water change. Then the result is 0.9(2500) + 0.1(2700) = 2520 ppm.
No ... i do not suggest anythingAre you suggesting that the BRS Fauna Balling Lite product should only be used by folks using certain salts that start with excessive sulfate?
But it's either using salt with higher levels of sulfate, or using another source like MgSO4 to keep the sulfates in line with the rest.
Besides that; SO4 can also be found as an impurity in CaCl2 2H2O (0.02%) MgCl2 6H2O (0.01 %) and NaHCO3 (0,01%)
These numbers are from the v.p. grade chemicals I use ( v.p. means very pure)
But I don't know whether it's soluble SO4 in CaCl or something like CaSO4.
Chloride is the most abundant ion in seawater, and sulfate is third. So the ratio between the two is not something that should vary all that much between quailty salt mixes. It should not vary at all in mixes that claim to provide NSW concentrations of ions. But however much it varies, a water change serves to bring it back toward that starting point by the amount I calculated. 
No one does DIY tests for chloride or sulfate, but labs do if one wants that.
No one does DIY tests for chloride or sulfate, but labs do if one wants that.
Ok I have hplc test results for Royal nature salt. What is the ratio of chloride to sulphate parts from say, the Great Barrier Reef? Or is it the same all over the world?
The ratios of the major ions are unchanged in the oceans, except near river mouths or in some closed environments.
By weight sulfate is third, but with 55.3 mM/l magnesium is third. Sulfate counts almost half the amount with 28 mM/l
That is certainly true. I show that in my article on what is in seawater:
What is seawater
http://reefkeeping.com/issues/2005-11/rhf/index.php
from it:
Figure 3. Relative concentration of ions in seawater by weight.
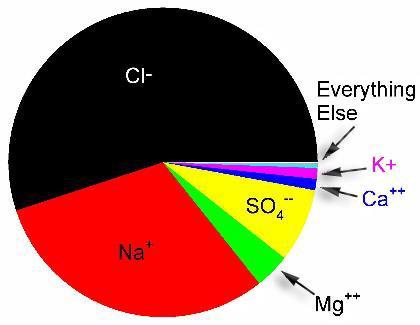
Figure 4. Relative concentration of ions in seawater by number.
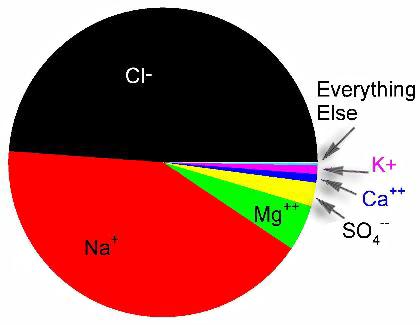
The ratios of the major ions are unchanged in the oceans, except near river mouths or in some closed environments.
By weight sulfate is third, but with 55.3 mM/l magnesium is third. Sulfate counts almost half the amount with 28 mM/l
That is certainly true. I show that in my article on what is in seawater:
What is seawater
http://reefkeeping.com/issues/2005-11/rhf/index.php
from it:
Figure 3. Relative concentration of ions in seawater by weight.

Figure 4. Relative concentration of ions in seawater by number.

Now I remember, that's where I got it from a few years ago.
I just couldn't get my head around this, and that article triggered my insatiable hunger for everything there is to know about seawater
I even "analyzed" Frank Milleros' artificial salt which b.t.w. is unbelievably balanced.
sum anions in mM x their valence = sum cations in mM x their valence, and the difference is only 0.00007
I just couldn't get my head around this, and that article triggered my insatiable hunger for everything there is to know about seawater
I even "analyzed" Frank Milleros' artificial salt which b.t.w. is unbelievably balanced.
sum anions in mM x their valence = sum cations in mM x their valence, and the difference is only 0.00007
Last edited:
:thumbsup:
Happy Reefing.
Happy Reefing.
Similar threads
- Replies
- 172
- Views
- 7K
- Replies
- 17
- Views
- 2K

