saltyclaus
New member
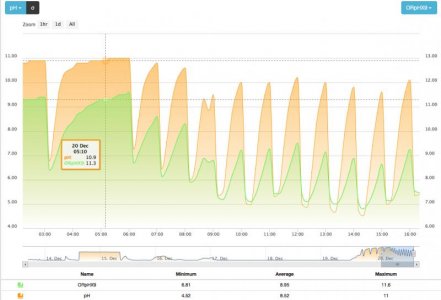
Ah, good point. I was thinking the knee was the point where oxygen was depleted and the filter was transitioning to nitrate. But it is actually the point where nitrate is exhausted and the bacteria are standing in front of the open cupboard and wondering what else is there to eat?
More common ground
Yes, based on the fact the knee signals that sulfate reduction has started, it is very beneficial to end the cycle as soon as a knee is confirmed.
That is what I believe will be the biggest challenge in getting the algorithm correct. Making it able to adapt to the nutrient loading of the system. Being able to establish the amount of N by the time period it takes to reduce it would be very helpful. The DyMiCo manual stresses the need for the flush pump to be restricted to a specific flow range. I assume this is because they are doing something similar.
Also they (DyMiCo), state that the max on time for the flsuh pump is 16 minutes per hour. I am guessing that this is tied to the max processing ability for their fully cycled filter running at 100% efficiency. The fow rate the user is instructed to restrict the flush pump is different between the model 700 & 2000 filter, so this cap of 16 minutes of flush pump activation can be true for both filter models as they are returing water at different rates. This could also be a potential signal for adjusting the operation of the filter. If we can establish the limit of the processig capabilities of an NPR of a given size, the algorithm could attempt to shorten the cycle period until the limit is reached based on the flow rate. Then again what you have already proposed may reach this same end point in a more elegant fashion.
I presume as well that the reason is to be sought in their algorithm. The two other reasons I mentioned before might come into play too but the flow seems overly emphasized in the manual if those were the only reasons (plankton preservation and preventing filter going aerobic).
Hence volume being a factor in their algorithm seems very plausible but so far I don't see a reason why it would be necessary. I've started to think about the logic flow diagram for the algorithm and in a nutshell I think about something as simple as:
1. determine the minimum ORP point
2. determine the maximum ORP point
3. flush the filter X seconds
4. wait for ORP peak
5. adapt X if ORP goes above ORP high target (1)
5. dose Y ml of carbon
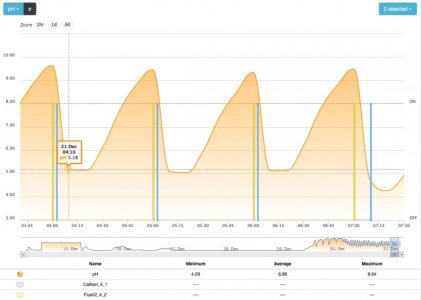
6. wait for nitrate knee or accelerated descent or ORP minimum
7. adapt Y depending on time between 3 and 6 versus previous cycles (2)
8. once a week goto 1 else goto 3
(1) high target is a function of the ORP range and empiric constants
(2) adaptation depends on sign of factor and an empiric constant z:
if at start or reset necessary (once a week/month/...)
Y = 0
factor = +1
else if time is equal within boundaries or time > previous
factor = factor x -1 (invert)
Y = Y + (factor x z)
The above has two evolutive parts:
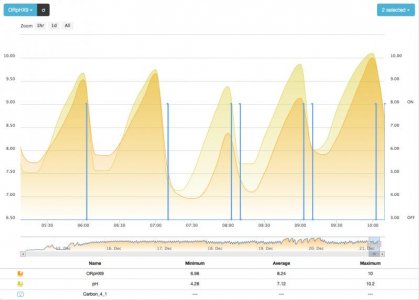
- the time to flush the filter which evolves with the resulting ORP after a cycle (lower ORP at the end will yield a longer flush next cycle)
- the dosed C evolves with the length of the cycle:
an equal time result in a higher or lower C depending on what happened last cycle
a longer time and we were augmenting C = diminish C
a longer time and we were diminishing C = augment C
that way the algorithm aims for a C in balance but once found continuously moves C up and down to allow it to drift if the bio-load changes
To be sure that the amount of C dials in correctly a reset happens periodically and the system dials the C back in
With this evolutive adaptation I imagine the filter should be able to adapt to any flow rate, filter volume, tank volume, media bio-mass capacity, bio-load and so on.
This is just a first quick brainstorm on how it could function. It needs fine tuning, safeguards and analytical routines which I'm pondering about. I'll make a flowchart once most of it sorts out.
In addition to the primary nutrients, N & P, it would be very helpful if the filter can also impact DOC. Some of these compounds will likely be dealt with in due course of normal filter operations. Others might require some encouragement for the bacteria to reduce them. The DyMiCo manual mentions that the plankton that are now able to survive and thrive can reduce the DOC. I am not aware of any tests for DOC available for hobbyists. I guess we could develop a visual test, kind of like the secchi sticks for estimating phyto plankton density. Only this would be for detecting the yellowing of the tank water from DOC by viewing 2 side by side vessels (1 tank, 1 RODI) from above over a background of varying shades of yellow (for the vessel filled with RODI).
Anyway best learn to walk before we run.
Dennis
One of the studies that I linked before was about forgoing an external carbon source and using the waste water as source of C (to diminish operational costs). They were able to accomplish similar denitrification so it sure seems like a possibility. They didn't mention what they thought the bacteria fed on but DOCs seems like the only candidate (certainly not POCs). Of course this necessitates that the bacteria are C deficient as the ethanol or acetate added as external source would surely be preferred opposed to consuming complex molecules such as amino acids or sugars. Hence another important reason to optimize the C dosage in function of denitrification and aim for that balance. Another idea might be to occasionally adapt a cycle to skip the C dosage, dose a lower amount or dose later to induce starving bacteria that go for DOCs in the cupboard. Anyways, this will require copious field testing and surely falls in the category "˜running' opposed to "˜walking' ;-)
Kr,
Klaus