You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
pH ;how to manage it; how not to manage it
- Thread starter tmz
- Start date
Good Morning and Good News...
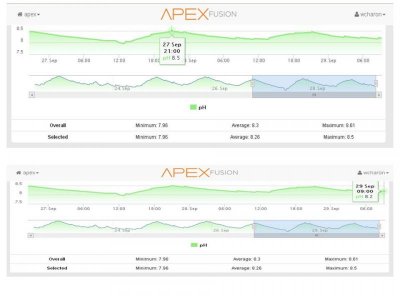
To keep updated i have switched from Baked Baking Soda to regular Baking Soda and my PH has stabilize a lot. The parameters fluctuations has been from 8.19 thru 8.5 the most as shown in the graph in the past days from 8.62.
Will keep an eye to see how it goes but next step will be to lower a little the strength of the KALK thru ATO to see if i can lower it a little more to something around 8.4.
Thanks for all the advise.
To keep updated i have switched from Baked Baking Soda to regular Baking Soda and my PH has stabilize a lot. The parameters fluctuations has been from 8.19 thru 8.5 the most as shown in the graph in the past days from 8.62.
Will keep an eye to see how it goes but next step will be to lower a little the strength of the KALK thru ATO to see if i can lower it a little more to something around 8.4.
Thanks for all the advise.
Attachments
cardiffgiant
Active member
Also curious, how old is your PH probe and when was the last time htat it was cleaned/calibrated?
When you add baked baking soda/ soda ash(CO3), it gives double the alkalinity of unbaked baking soda (HCO3). The higher alk will raise pH for a short time ( usually an hour or two) but CO2 from the air will bring it back down leaving increased alkalinity and about the same low pH. When you add HCO3 at double the volume of CO3 you are adding the same amount of alkalinty but also adding H which results in a lower pH effect.
Increasing alkalinity to manage pH is usually a poor strategy since bouncing alk levels are harmful to organisms that calcify like corals and high alk often leads to precipitation of calcium carbonate as well. It's fine to increase alk when it's low at the start though but the effects on pH will be mostly transient and brief.
Increasing alkalinity to manage pH is usually a poor strategy since bouncing alk levels are harmful to organisms that calcify like corals and high alk often leads to precipitation of calcium carbonate as well. It's fine to increase alk when it's low at the start though but the effects on pH will be mostly transient and brief.
Last edited:
Also curious, how old is your PH probe and when was the last time htat it was cleaned/calibrated?
My PH probe is new and only 3 weeks. It was calibrated with 7 and 10 ph solutions sealed. Also double checked with Salifert test.
When you add baked baking soda/ soda ash(CO3), it gives double the alkalinity of unbaked baking soda (HCO3). The higher alk will raise pH for a short time ( usually an hour or two) but CO2 from the air will bring it back down leaving increased alkalinity and about the same low pH. When you add HCO3 at double the volume of CO3 you are adding the same amount of alkalinty but also adding H which results in a lower pH effect.
Increasing alkalinity to manage pH is usually a poor strategy since bouncing alk levels are harmful to organisms that calcify like corals and high alk often leads to precipitation of calcium carbonate as well. It's fine to increase alk when it's low at the start though but the effects on pH will be mostly transient and brief.
Yes TMZ you are correct. Actually i lowered my ALK dosing rate. My parameters reading from yesterday was ALK 8.7, Calcium 425, Mg 1400.
So i think i am in a good route now. I will be targeting for:
ALK 8 - 8.5
Calcium - 415 - 425
Mg - 1380 - 1420
PH - 8.1 - 8.4
Mental1
New member
My pH is, at the moment around 7.4. I am dosing soda ash at night for about ten minutes. It raised it somewhat (it was at 7.08). What's crazy is that I have lots of surface agitation, lots of tumbling water, not a lot of teenagers breathing in the house. I haven't checked Alk yet, this has just been on for the last two nights. How quickly can you raise the pH? I haven't tried fresh air into the skimmer but the skimmer is in the basement so maybe not a lot of fresh air going in there during the winter. IN addition, I have been wrestling with my system for the last year, it almost completely crashed. All the sand has been replaced and a lot has been cleaned out. Now I just have this last piece left -- chronically low pH.
Capt_Cully
Active member
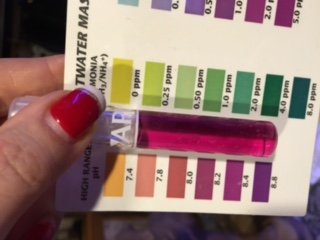
What are you using to measure pH? Have you verified it with other methods or test kits?
It is crazy low. I doubt 7.08 is even possible in salt water. What is the salinity ;alkalinity? coral skelton, mollusk shells and anything else made of argontie like live rock and aragonite sand begin to dissolve at/ under 7.7pH. When they do they not only kill the organisms they also add alkalinity which mediated the pH.
Mental1
New member
I haven't tested everything else since I got this reading. I will do it tonight when I get home. I have had so many problems it is crazy. The topoff hasn't been properly reset up since I sent the controller off for a second time. I drowned the tank so I am sure salinity is low at them moment. There are not many live corals in my tank. Softies do ok. Fish are ok. I have lots of red algae that grows beautifully. It's been a long slow recovery. I never had problems with pH prior to this all happening. I also have a weird bacterial growth. SO much fun.
Mental1
New member
I really don't know what to do. I am going to stop dosing because the ALK is too high. My husband is going to run a hose from outside to the skimmer -- it involves drilling through the sill of the house as the skimmer is in the basement. I am at a loss but will send these results to DA...
cardiffgiant
Active member
How old is the RKe probe and when was the last time that you cleaned and calibrated it?
*edit - I just realized that I asked the exact same question to a different poster on the previous page. I was getting some weird readings recently and cleaned/calibrated, and all was right with the world. I also started using a 2nd PH probe instead of a ORP probe on my apex.
*edit - I just realized that I asked the exact same question to a different poster on the previous page. I was getting some weird readings recently and cleaned/calibrated, and all was right with the world. I also started using a 2nd PH probe instead of a ORP probe on my apex.
Last edited:
Capt_Cully
Active member
I would definitely correlate with a different probe set up or test kits. It sure sounds low.
cardiffgiant
Active member
Is it a new probe? Was it out of the tank or storage solution for any duration of time? Using soda ash, having high alk, and getting an expected reading with the API test all points to a bad measurement from the RKE or probe.
The api results are more in line with what I'd expect with dkh at 13. If the unit you have isn't defective , it could be electrical interference affecting the reading particularly when the lights are on. Sometimes , monitors and probes misread when ballasts are on and cables are close together or when they share the same outlet power source.
An outside airline needs to be large enough to allow free air flow ; larger diameter than the skimmer air intake hose if it's a long run.
An outside airline needs to be large enough to allow free air flow ; larger diameter than the skimmer air intake hose if it's a long run.
Increasing alkalinity to manage pH is usually a poor strategy since bouncing alk levels are harmful to organisms that calcify like corals and high alk often leads to precipitation of calcium carbonate as well. It's fine to increase alk when it's low at the start though but the effects on pH will be mostly transient and brief.
doesn't high alk levels need high calcium levels too to form calcium carbonate to participate?
Similar threads
- Replies
- 0
- Views
- 774
- Replies
- 0
- Views
- 217
- Replies
- 1
- Views
- 698