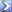It's tough to take a good picture of my actual system, but here's the basic gist from a file photo:

The skimmer is the two narrow chambers on the left, separated by the vertical baffles. The entire system is fed by an EcoPlus pump fitted with a meshwheel impeller, connected to the black tube on the far left. I dose the LaCl directly into the impeller intake.
Underneath the skimmer cup, I have a Hydor Ario aeration pump aimed into the first chamber, to chop up more bubbles and add some recirculation.
From what I can tell the LaCl settles in the first and second chamber, and any overspill lands in the big refugium trough. In my system it is barebottom and stuffed with chaeto, lit by a single T5 at 6700k. You'll notice two holes that allow water into the refugium trough. I have the bottom hole covered with a credit card, secured by a magnet. This raises the water level in the entire skimmer, adding water volume and creating more vertical space to baffle any LaCl precipitate. I think it works, because I have never noticed any significant water clouding in my refugium. I also keep a few corals in there (xenia, frogspawn, pocillopora) and none have ever looked bothered.
I haven't yet felt the need to vaccuum out the sediment, I can see a little bit down there, though.
I also run a TLF150 full of PO4 adsorption resin, but the LaCl is so cost effective I think I'm going to turn it into a purigen reactor.

The skimmer is the two narrow chambers on the left, separated by the vertical baffles. The entire system is fed by an EcoPlus pump fitted with a meshwheel impeller, connected to the black tube on the far left. I dose the LaCl directly into the impeller intake.
Underneath the skimmer cup, I have a Hydor Ario aeration pump aimed into the first chamber, to chop up more bubbles and add some recirculation.
From what I can tell the LaCl settles in the first and second chamber, and any overspill lands in the big refugium trough. In my system it is barebottom and stuffed with chaeto, lit by a single T5 at 6700k. You'll notice two holes that allow water into the refugium trough. I have the bottom hole covered with a credit card, secured by a magnet. This raises the water level in the entire skimmer, adding water volume and creating more vertical space to baffle any LaCl precipitate. I think it works, because I have never noticed any significant water clouding in my refugium. I also keep a few corals in there (xenia, frogspawn, pocillopora) and none have ever looked bothered.
I haven't yet felt the need to vaccuum out the sediment, I can see a little bit down there, though.
I also run a TLF150 full of PO4 adsorption resin, but the LaCl is so cost effective I think I'm going to turn it into a purigen reactor.
Last edited: