You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
White fuzz on live rock
- Thread starter mtb888
- Start date
HighlandReefer
Team RC
nobody huh?
You will need to look at this organism under the scope to determine what it is. After your wife makes an ID, I would be interested in hearing the results and seeing any micro pictures she can provide.
Cooking rock will not necessarily kill this organism. I would bleach the rock several times to kill it, if you decide to resort to drastic measures.
ExFOWLR
New member
She won't be able to get a picture because technically she isn't supposed to be looking at/testing hubby's marine aquarium stuff. She has her own scope in her office that she can use but the bazillion dollar one with the camera is located in the main lab and could provoke questions.
Actually now my wife has offered to "show me her work" today and may have access to the camera. How cool is that? She has only been there 5 years and I have never been there. If I can get pics I will post them because ultimately the goal is to ID the organism(s) and destroy them.
Cliff you seem to know this sort of stuff intimately and she was wondering if you knew whether these would be "gram pos. or negative" or which stains you would use. At the same time we still do not know that the organism(s) are. When she scoped them the other day unstained she saw dozens of different things she desribed as diatoms, protozoa, bacteria, and the dominant one wrere strings of rectangular shaped (bacteria?,fungus?) with a definate nucleus in each. This is what I believe is what can be seen in my crappy pictures. If you have any advice or insight on identifying this would be highly appreciated. hopefully you see this post today because we are trying to go there this afternoon when we can get a babysitter and possibly run a sample through the chemistry analyzer as well.
Thanks for your help, John
Actually now my wife has offered to "show me her work" today and may have access to the camera. How cool is that? She has only been there 5 years and I have never been there. If I can get pics I will post them because ultimately the goal is to ID the organism(s) and destroy them.
Cliff you seem to know this sort of stuff intimately and she was wondering if you knew whether these would be "gram pos. or negative" or which stains you would use. At the same time we still do not know that the organism(s) are. When she scoped them the other day unstained she saw dozens of different things she desribed as diatoms, protozoa, bacteria, and the dominant one wrere strings of rectangular shaped (bacteria?,fungus?) with a definate nucleus in each. This is what I believe is what can be seen in my crappy pictures. If you have any advice or insight on identifying this would be highly appreciated. hopefully you see this post today because we are trying to go there this afternoon when we can get a babysitter and possibly run a sample through the chemistry analyzer as well.
Thanks for your help, John
HighlandReefer
Team RC
"Cliff you seem to know this sort of stuff intimately and she was wondering if you knew whether these would be "gram pos. or negative" or which stains you would use."
I'm not familiar with all the different stains and techniques used to help ID the various bacteria, algae, cyano, molds......etc. Needless to say it does get complicated. Most microbiologists specialize in one specific grouping usually and may know little about other groups and techniques used to help ID them.
I'm not familiar with all the different stains and techniques used to help ID the various bacteria, algae, cyano, molds......etc. Needless to say it does get complicated. Most microbiologists specialize in one specific grouping usually and may know little about other groups and techniques used to help ID them.
HighlandReefer
Team RC
The biggest problem once you properly ID your pest, is that there are few chemical control measures available to eradicate the pest. Hence the importance in not allowing pests to be introduced into your tank in the first place.
Basically the only products available to control pests in a reef tank are erythromycin and AlgaeFix. Other than that most rely on limiting phosphate, nitrate and dissolved organics. Many of these pests can survive at lower nutrient levels than can our coral. At that point you are left with weeding the pest out similar to weeding a flower garden.
Basically the only products available to control pests in a reef tank are erythromycin and AlgaeFix. Other than that most rely on limiting phosphate, nitrate and dissolved organics. Many of these pests can survive at lower nutrient levels than can our coral. At that point you are left with weeding the pest out similar to weeding a flower garden.
ExFOWLR
New member
My wife is a pretty smart cookie and can figure out the stains but it may take a little time. I will be heading to her lab in a couple hours and see what we can find. I don't discount the possibility that it is a completely common well known inhabitant and I just don't know what I'm looking at or doing. I think whatever is covering the rock may not have been an introduced pest because it started shortly after the live rock cured, before much if anything was introduced. It grows on hard surfaces in the display tank but not on the sand. it doesn't grow in the sump where I have a 6500k light and some cheato. It coveres the rock and gives it a green hue but looks opaque white in the light. it prevents coraline algae from growing and likes to grow on top of coraline that grew on plumbing and my overflow box. I think its also possible its some type of micro algae or multiple organisms. hopefully will have a few answers and possibly some pics soon.
HighlandReefer
Team RC
Sounds good.
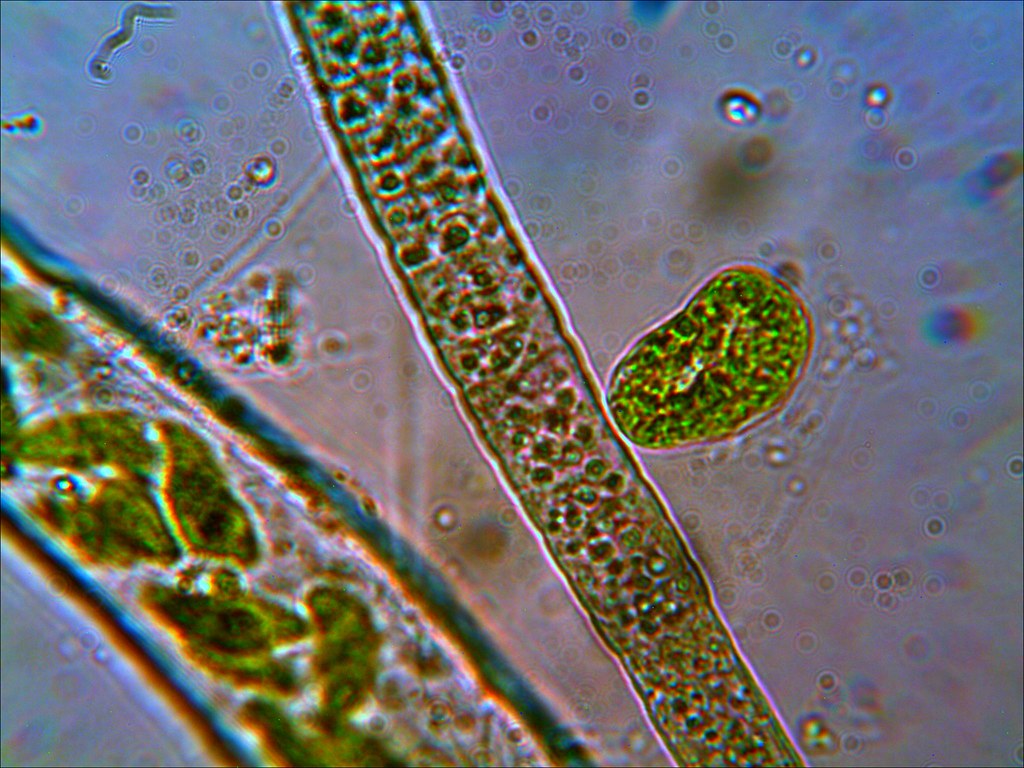
My bet is on a type of algae since it does have a green hue in color. Perhaps a cyano, would be my next bet.
The size of the cells can help determine between algae, cyano and bacteria.
You really need to bump the magnification up to see bacterial cells as more or less large dots.
The chloroplasts in algae are clearly evident. Cyanobacteria do not have chloroplasts but have their photosynthesizing pigments spread throughout the cell and not within a special membrane structure like algae (chloroplast).
Algae on the left & Cyanobacteria on the right (you can distinctly see the large chloroplasts in the algae on the left):
My bet is on a type of algae since it does have a green hue in color. Perhaps a cyano, would be my next bet.
The size of the cells can help determine between algae, cyano and bacteria.
You really need to bump the magnification up to see bacterial cells as more or less large dots.
The chloroplasts in algae are clearly evident. Cyanobacteria do not have chloroplasts but have their photosynthesizing pigments spread throughout the cell and not within a special membrane structure like algae (chloroplast).
Algae on the left & Cyanobacteria on the right (you can distinctly see the large chloroplasts in the algae on the left):

Last edited:
ExFOWLR
New member
photos
photos
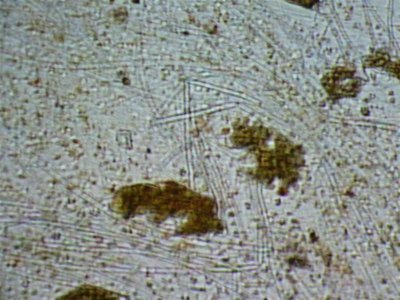
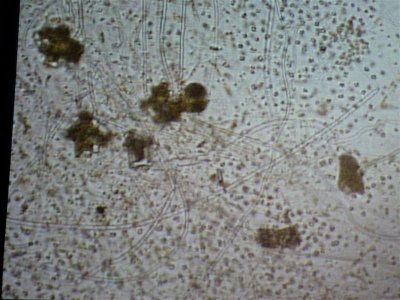
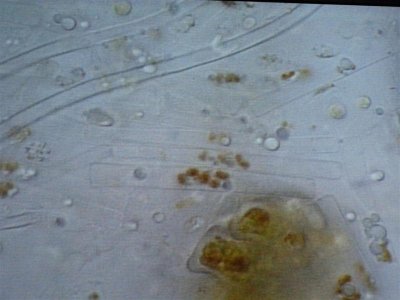
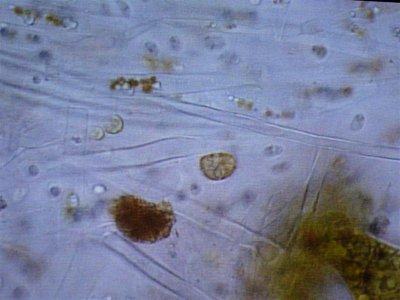
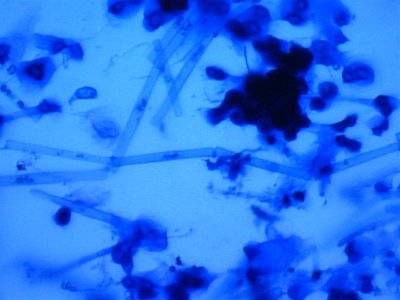
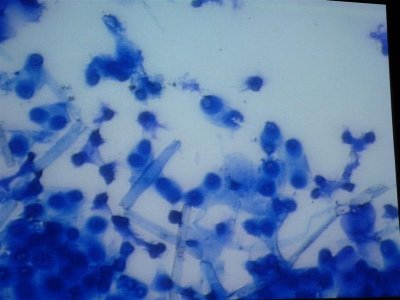
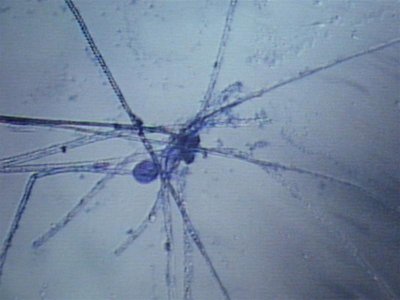
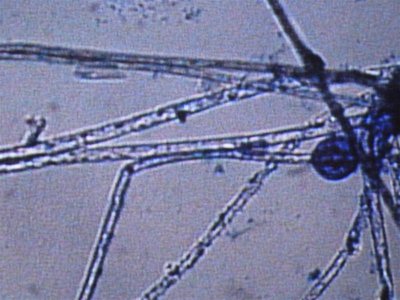
Here are some photos we got today. the quality isn't the best but it was the best we could do. I took in two different samples. One was from the glass and return manifold (same stuff) and the second sample is what was on the rock.
The first and second is a wet prep at 200X and the third and fourth are 1000X.
I actually have about 20 pics but not sure how to load all at the same time with captions so I may wait a bit until someone could tell me how.
photos
Here are some photos we got today. the quality isn't the best but it was the best we could do. I took in two different samples. One was from the glass and return manifold (same stuff) and the second sample is what was on the rock.
The first and second is a wet prep at 200X and the third and fourth are 1000X.
I actually have about 20 pics but not sure how to load all at the same time with captions so I may wait a bit until someone could tell me how.
Attachments
HighlandReefer
Team RC
Looks like a fungus to me. 
Here is an example of a fungus to compare to (this picture may be at more like 1500X to 2000X):
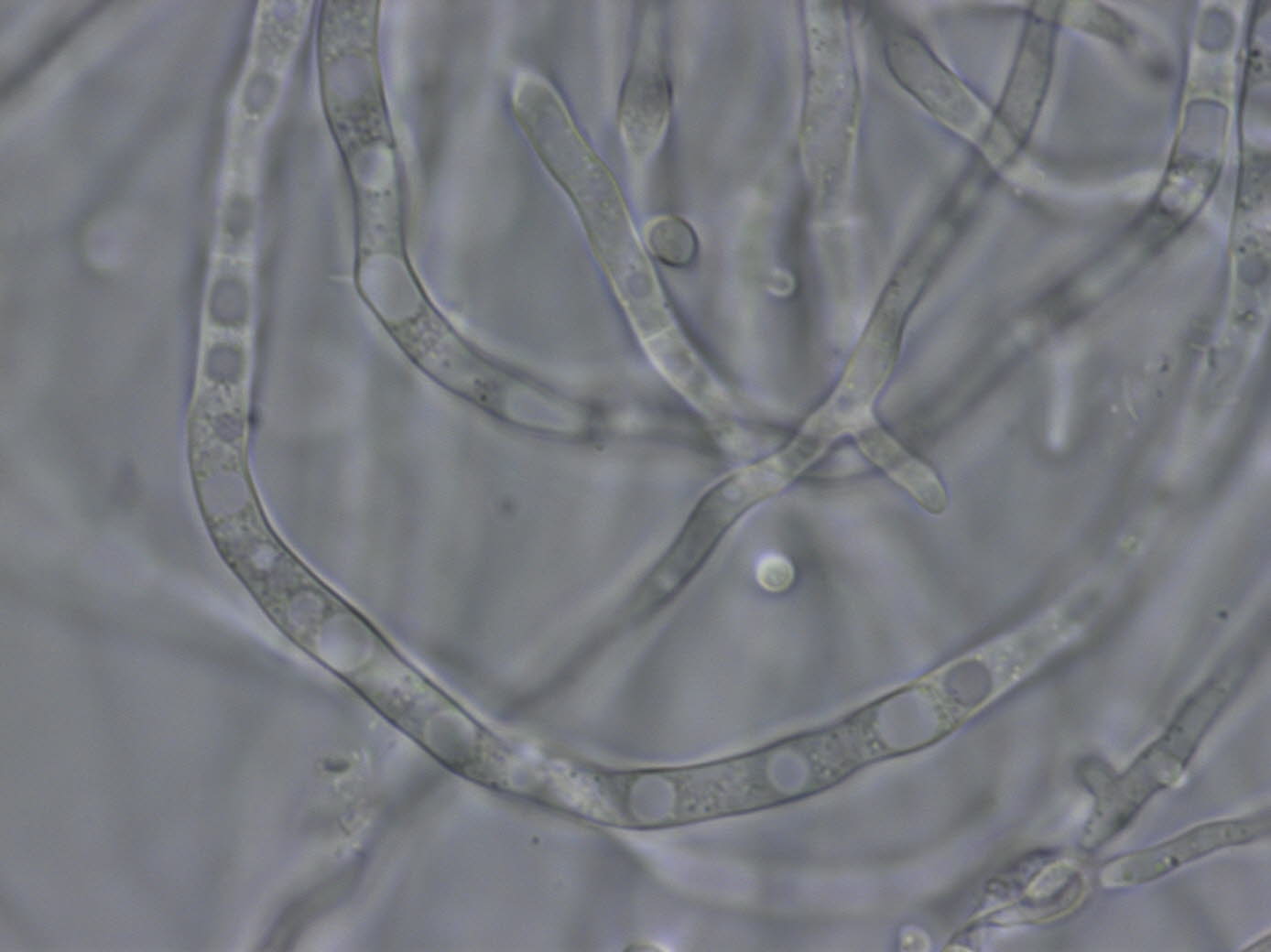
Here is an example of a fungus to compare to (this picture may be at more like 1500X to 2000X):

Last edited:
HighlandReefer
Team RC
An article of interest:
Use of Baking Soda as a Fungicide
George Kuepper, Raeven Thomas,
and Richard Earles
NCAT Agriculture Specialists
© NCAT November 2001
IP130 A printable PDF version of this entire document is available at:
http://attra.ncat.org/attra-pub/PDF/bakingsoda.pdf
4 pages — 367K
--------------------------------------------------------------------------------
Abstract
There has been considerable interest in the use of baking soda (sodium bicarbonate, NaHCO3) and potassium bicarbonate (KHCO3) to control powdery mildew and other fungal diseases of plants. This publication provides a brief survey of observations, research, and recommendations on the use of bicarbonates in horticulture.
The use of baking soda as a fungicide is not a new idea. In Alfred C. Hottes' A Little Book of Climbing Plants, published in 1933 by the A.T. De La Mare Co. of New York, mention is made of using one ounce of baking soda per gallon of water to control powdery mildew (PM) on climbing roses. The author credits the idea to a Russian plant pathologist, A. de Yaczenski.(1)
In the August, 1985 issue of Organic Gardening magazine, a short article by Warren Shultz entitled "Recipe for Resistance" reports that researchers in Japan obtained effective control of PM on cucumbers, eggplants, and strawberries. They suggested weekly sprays of ¼ ounce baking soda per gallon of water.(2)
An article in the June, 1990 issue of Greenhouse Manager magazine summarizes the results of three years of testing baking soda as a fungicide for roses. Cornell University researcher Dr. R. Kenneth Horst observed suppression of PM and blackspot—both major problems for New York rose growers. Roses were sprayed every 3 to 4 days with a water solution of baking soda and insecticidal soap. The latter was included for its surfactant qualities. (Surfactants are chemical agents that alter the surface properties of a liquid.) The soap improved the effectiveness of the bicarbonate by making it stick to, and spread evenly over, the leaf surface. Further experimentation proved that the insecticidal soap itself was not responsible for suppressing the diseases. While no specific concentration of baking soda is indicated as being most effective in PM suppression, the article states that a 0.5% solution was most effective in preventing blackspot.(3)
Some of the work at Cornell has focused on controlling fungal diseases on cucurbits.(4) A single spray application (to runoff) of 0.5% (wt./vol. of water) baking soda, plus 0.5% (vol./vol. of water) SunSpray UFP® horticultural oil almost completely inhibited PM on heavily infected pumpkin foliage. Baking soda without spray oil was ineffective, and a 2% (wt./vol. of water) solution of baking soda damaged the leaves. Baking soda/oil sprays also provided good control of urocladium leaf spot in cucumber, alternaria leaf blight in muskmelon, and gummy stem blight in muskmelon.(5) Other diseases against which baking soda may prove effective include anthracnose in cucurbits (6); rust, dollar spot, and pythium blight in turf; late blight in potato; rust in wheat; and diseases affecting peanuts, banana, and alfalfa.(7)
Researchers in Israel reported the successful use of baking soda and SunSpray oil in controlling PM on euonymus.(8) In this research a 2% baking soda and 1% oil solution proved most effective.(9)
On-farm observations on melon acreage in Virginia resulted in one farm operation switching from synthetic fungicides to a baking soda/oil spray. These growers incorporated a liquid fertilizer into the mix.(10)
Research in Germany evaluated baking soda as a control for PM on `Bacchus' grapes. Three spray applications were made, beginning when symptoms first appeared. Good control was achieved with no loss of grape quality. The optimum concentration was a 1% solution.(11)
An article in the February, 1996 issue of GrowerTalks magazine follows up on the continuing research at Cornell. Testing with a variety of bicarbonates revealed that selecting the correct bicarbonate for a particular disease is important. Dr. Horst's research team found that ammonium bicarbonate had the strongest effect on some diseases, while potassium and sodium bicarbonates worked best against others. Potassium bicarbonate provided the best control of PM. "Sodium bicarbonate is okay, but it's not as good," Horst is quoted as saying. "And ammonium bicarbonate doesn't do the job on powdery mildew." He points out that while conventional chemical controls for PM are preventatives only, bicarbonates can eliminate the disease after it has already appeared on certain crops—he mentions roses and an unspecified ornamental—provided the infection is not severe. The only plant damage associated with bicarbonates applied in the trials was foliar burning when application rates exceeded recommended concentrations. Testing established that sodium bicarbonate does not increase the levels of sodium in plant tissues, soil, or runoff water. While their precise mode of action against fungi is not understood, Horst states that bicarbonates seem to damage the cell wall membrane in PM spores. He also believes pH to be a factor in bicarbonate effectiveness. In any case, bicarbonates are contact fungicides, and kill PM within minutes.(7)
The Federal EPA ruled (as of December, 1996) that sodium and potassium bicarbonates are exempt from residue tolerances.(12) This action served to facilitate the development and release of commercial bicarbonate products for horticultural use. It also lent weight to the belief that these materials are largely innocuous from a food safety perspective.
Connecticut researchers evaluated the effects of a spray solution containing 1% each of baking soda and horticultural oil on PM infection in zucchini, pumpkin, and cantaloupe. Four applications were made and disease suppression was definitely observed, accompanied by reduced insect pest damage. These researchers maintain that the treatment is preventative—not curative; that it is only necessary in years where early outbreaks may threaten yields; and that spraying should accompany proper nutrition and water management.(13)
In 1998, Church & Dwight Co. (14)—the manufacturer of Arm & Hammer™ baking soda—received EPA registration for Armicarb 100®, a potassium bicarbonate formulation, for use against PM, downy mildew, botrytis, and alternaria leaf-spot.(15) This product is the direct result of Dr. Horst's research at Cornell, which was funded by Church & Dwight. Armicarb 100 is now available from Helena Chemical Company.(16) A similar product is sold under the name FirstStep® by the W.A. Cleary Chemical Co.(17)
The EPA and the California Department of Environmental Protection have provided registration to Monterey Chemical Co. (18) for a product called Kaligreen®. A potassium bicarbonate fungicide for PM control, it is cleared for use on grapes, cucumbers, tobacco, roses, strawberries, and a wide range of other crops.(19, 20, 21) Directions for use include the addition of a sticker-spreader surfactant and a caution against use in acidic spray mixes. Since the product contains 30% potassium it is also touted for its fertilizer value.(22) One source of Kaligreen® is Peaceful Valley Farm Supply.(23)
Yet another potassium bicarbonate product, Remedy®, by Bonide™ (24), is now available from Gardener's Supply Co.(25) This formulation, which includes a surfactant oil, is labeled for use on ornamental, nut, and fruit trees, shrubs, and many vegetable plants. Said to control PM, black spot, leaf spot, anthracnose, phoma, phytophthora, scab, botrytis, and many other diseases, Remedy is particularly targeted toward rose growers.(26)
Various carbonates and bicarbonates have been proven effective against gray mold, the number one post-harvest disease of grapes. Researchers found that carbonates were more effective than bicarbonates at reducing gray mold (Botrytis cinerea) spore germination, and that sodium and ammonium bicarbonates were better than potassium bicarbonate.(27)
While industry was in the process of developing bicarbonate products for commercial and home horticulture, a number of recommendations for using kitchen-grade baking soda surfaced in print. These include:
•J. Howard Garrett—a well-known horticultural columnist and radio personality in the Dallas, Texas, area—recommends baking soda sprays at a concentration of 4 teaspoons per gallon of water for control of PM, blackspot, brown patch, and other fungal diseases. He also suggests that a light soil spray of baking soda solution can suppress fungus gnat problems, while cautioning that overuse should be avoided because of possible negative effects (sodium accumulation and alkaline pH) on the soil.(28)
•The authors of an organic pest control handbook suggest the same concentration mixture as Garrett, but advise the addition of an equal quantity of liquid dish soap or insecticidal soap as a surfactant.(29)
•The P. Allen Smith Gardens website advises mixing 1 heaping tablespoon of baking soda, 1 tablespoon of dormant oil, and ½ teaspoon of insecticidal or dish soap in one gallon of water as a PM spray. Stating that plants should be well hydrated prior to spraying, this source recommends irrigating a couple days in advance.(30)
Use of Baking Soda as a Fungicide
George Kuepper, Raeven Thomas,
and Richard Earles
NCAT Agriculture Specialists
© NCAT November 2001
IP130 A printable PDF version of this entire document is available at:
http://attra.ncat.org/attra-pub/PDF/bakingsoda.pdf
4 pages — 367K
--------------------------------------------------------------------------------
Abstract
There has been considerable interest in the use of baking soda (sodium bicarbonate, NaHCO3) and potassium bicarbonate (KHCO3) to control powdery mildew and other fungal diseases of plants. This publication provides a brief survey of observations, research, and recommendations on the use of bicarbonates in horticulture.
The use of baking soda as a fungicide is not a new idea. In Alfred C. Hottes' A Little Book of Climbing Plants, published in 1933 by the A.T. De La Mare Co. of New York, mention is made of using one ounce of baking soda per gallon of water to control powdery mildew (PM) on climbing roses. The author credits the idea to a Russian plant pathologist, A. de Yaczenski.(1)
In the August, 1985 issue of Organic Gardening magazine, a short article by Warren Shultz entitled "Recipe for Resistance" reports that researchers in Japan obtained effective control of PM on cucumbers, eggplants, and strawberries. They suggested weekly sprays of ¼ ounce baking soda per gallon of water.(2)
An article in the June, 1990 issue of Greenhouse Manager magazine summarizes the results of three years of testing baking soda as a fungicide for roses. Cornell University researcher Dr. R. Kenneth Horst observed suppression of PM and blackspot—both major problems for New York rose growers. Roses were sprayed every 3 to 4 days with a water solution of baking soda and insecticidal soap. The latter was included for its surfactant qualities. (Surfactants are chemical agents that alter the surface properties of a liquid.) The soap improved the effectiveness of the bicarbonate by making it stick to, and spread evenly over, the leaf surface. Further experimentation proved that the insecticidal soap itself was not responsible for suppressing the diseases. While no specific concentration of baking soda is indicated as being most effective in PM suppression, the article states that a 0.5% solution was most effective in preventing blackspot.(3)
Some of the work at Cornell has focused on controlling fungal diseases on cucurbits.(4) A single spray application (to runoff) of 0.5% (wt./vol. of water) baking soda, plus 0.5% (vol./vol. of water) SunSpray UFP® horticultural oil almost completely inhibited PM on heavily infected pumpkin foliage. Baking soda without spray oil was ineffective, and a 2% (wt./vol. of water) solution of baking soda damaged the leaves. Baking soda/oil sprays also provided good control of urocladium leaf spot in cucumber, alternaria leaf blight in muskmelon, and gummy stem blight in muskmelon.(5) Other diseases against which baking soda may prove effective include anthracnose in cucurbits (6); rust, dollar spot, and pythium blight in turf; late blight in potato; rust in wheat; and diseases affecting peanuts, banana, and alfalfa.(7)
Researchers in Israel reported the successful use of baking soda and SunSpray oil in controlling PM on euonymus.(8) In this research a 2% baking soda and 1% oil solution proved most effective.(9)
On-farm observations on melon acreage in Virginia resulted in one farm operation switching from synthetic fungicides to a baking soda/oil spray. These growers incorporated a liquid fertilizer into the mix.(10)
Research in Germany evaluated baking soda as a control for PM on `Bacchus' grapes. Three spray applications were made, beginning when symptoms first appeared. Good control was achieved with no loss of grape quality. The optimum concentration was a 1% solution.(11)
An article in the February, 1996 issue of GrowerTalks magazine follows up on the continuing research at Cornell. Testing with a variety of bicarbonates revealed that selecting the correct bicarbonate for a particular disease is important. Dr. Horst's research team found that ammonium bicarbonate had the strongest effect on some diseases, while potassium and sodium bicarbonates worked best against others. Potassium bicarbonate provided the best control of PM. "Sodium bicarbonate is okay, but it's not as good," Horst is quoted as saying. "And ammonium bicarbonate doesn't do the job on powdery mildew." He points out that while conventional chemical controls for PM are preventatives only, bicarbonates can eliminate the disease after it has already appeared on certain crops—he mentions roses and an unspecified ornamental—provided the infection is not severe. The only plant damage associated with bicarbonates applied in the trials was foliar burning when application rates exceeded recommended concentrations. Testing established that sodium bicarbonate does not increase the levels of sodium in plant tissues, soil, or runoff water. While their precise mode of action against fungi is not understood, Horst states that bicarbonates seem to damage the cell wall membrane in PM spores. He also believes pH to be a factor in bicarbonate effectiveness. In any case, bicarbonates are contact fungicides, and kill PM within minutes.(7)
The Federal EPA ruled (as of December, 1996) that sodium and potassium bicarbonates are exempt from residue tolerances.(12) This action served to facilitate the development and release of commercial bicarbonate products for horticultural use. It also lent weight to the belief that these materials are largely innocuous from a food safety perspective.
Connecticut researchers evaluated the effects of a spray solution containing 1% each of baking soda and horticultural oil on PM infection in zucchini, pumpkin, and cantaloupe. Four applications were made and disease suppression was definitely observed, accompanied by reduced insect pest damage. These researchers maintain that the treatment is preventative—not curative; that it is only necessary in years where early outbreaks may threaten yields; and that spraying should accompany proper nutrition and water management.(13)
In 1998, Church & Dwight Co. (14)—the manufacturer of Arm & Hammer™ baking soda—received EPA registration for Armicarb 100®, a potassium bicarbonate formulation, for use against PM, downy mildew, botrytis, and alternaria leaf-spot.(15) This product is the direct result of Dr. Horst's research at Cornell, which was funded by Church & Dwight. Armicarb 100 is now available from Helena Chemical Company.(16) A similar product is sold under the name FirstStep® by the W.A. Cleary Chemical Co.(17)
The EPA and the California Department of Environmental Protection have provided registration to Monterey Chemical Co. (18) for a product called Kaligreen®. A potassium bicarbonate fungicide for PM control, it is cleared for use on grapes, cucumbers, tobacco, roses, strawberries, and a wide range of other crops.(19, 20, 21) Directions for use include the addition of a sticker-spreader surfactant and a caution against use in acidic spray mixes. Since the product contains 30% potassium it is also touted for its fertilizer value.(22) One source of Kaligreen® is Peaceful Valley Farm Supply.(23)
Yet another potassium bicarbonate product, Remedy®, by Bonide™ (24), is now available from Gardener's Supply Co.(25) This formulation, which includes a surfactant oil, is labeled for use on ornamental, nut, and fruit trees, shrubs, and many vegetable plants. Said to control PM, black spot, leaf spot, anthracnose, phoma, phytophthora, scab, botrytis, and many other diseases, Remedy is particularly targeted toward rose growers.(26)
Various carbonates and bicarbonates have been proven effective against gray mold, the number one post-harvest disease of grapes. Researchers found that carbonates were more effective than bicarbonates at reducing gray mold (Botrytis cinerea) spore germination, and that sodium and ammonium bicarbonates were better than potassium bicarbonate.(27)
While industry was in the process of developing bicarbonate products for commercial and home horticulture, a number of recommendations for using kitchen-grade baking soda surfaced in print. These include:
•J. Howard Garrett—a well-known horticultural columnist and radio personality in the Dallas, Texas, area—recommends baking soda sprays at a concentration of 4 teaspoons per gallon of water for control of PM, blackspot, brown patch, and other fungal diseases. He also suggests that a light soil spray of baking soda solution can suppress fungus gnat problems, while cautioning that overuse should be avoided because of possible negative effects (sodium accumulation and alkaline pH) on the soil.(28)
•The authors of an organic pest control handbook suggest the same concentration mixture as Garrett, but advise the addition of an equal quantity of liquid dish soap or insecticidal soap as a surfactant.(29)
•The P. Allen Smith Gardens website advises mixing 1 heaping tablespoon of baking soda, 1 tablespoon of dormant oil, and ½ teaspoon of insecticidal or dish soap in one gallon of water as a PM spray. Stating that plants should be well hydrated prior to spraying, this source recommends irrigating a couple days in advance.(30)
Last edited:
ExFOWLR
New member
more photos
more photos
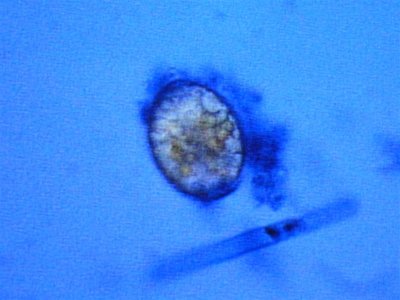
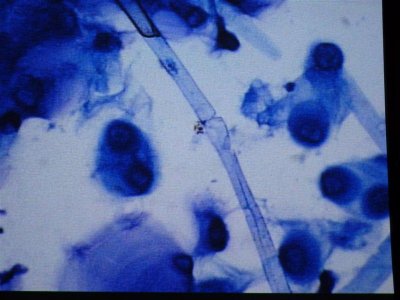
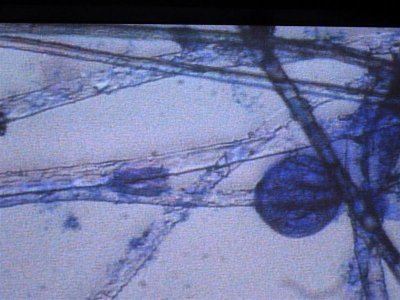
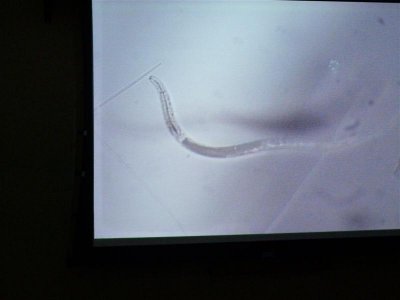
here are more photos some stained with methylene blue and others with a "wright" stain where acidic organisms turn blue and basic things turn pink.
The first is 400X in MB, the second is some type of egg at 1000X.
more photos
here are more photos some stained with methylene blue and others with a "wright" stain where acidic organisms turn blue and basic things turn pink.
The first is 400X in MB, the second is some type of egg at 1000X.
Attachments
ExFOWLR
New member
HighlandReefer
Team RC
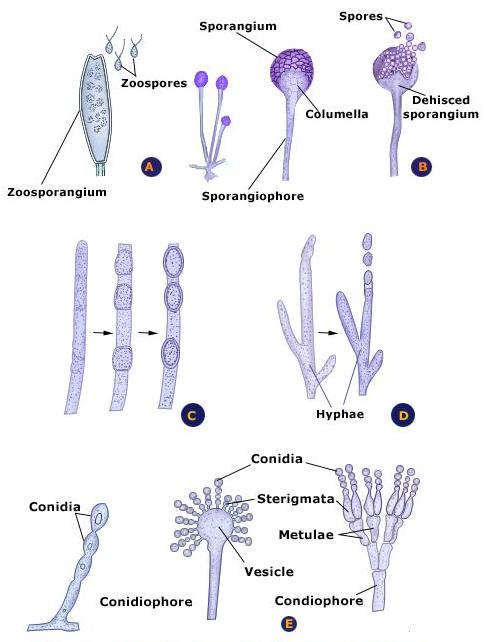
The last set of picture looks like one of the fungal spore types used for reproduction (perhaps conidial spores):

Last edited:
ExFOWLR
New member
sample 2
sample 2
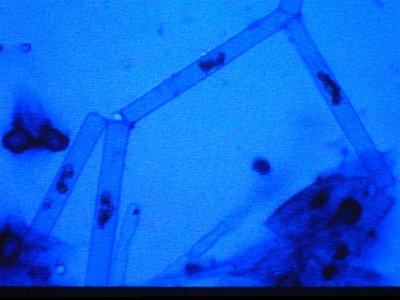
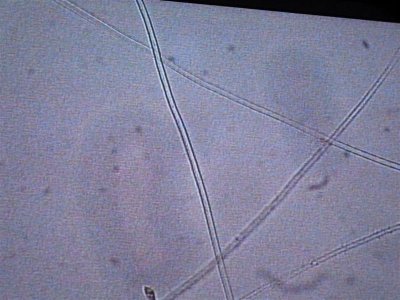
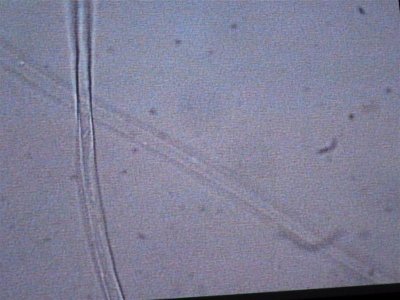
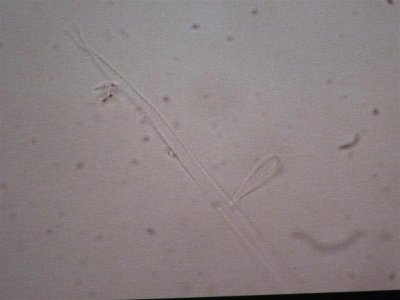
the next sample taken came right from the rock. I took a small piece of rock and brought it in a sample cup with tank water. these are the opaque white hairs that are present on the rock. Notice the complete lack of any cellular structure. Is this dead and was living at one time. It resembles GHA when you look at it but it is less than 1/4" tall and covers all the rock.
The first is at 200X, the second is 400X.
sample 2
the next sample taken came right from the rock. I took a small piece of rock and brought it in a sample cup with tank water. these are the opaque white hairs that are present on the rock. Notice the complete lack of any cellular structure. Is this dead and was living at one time. It resembles GHA when you look at it but it is less than 1/4" tall and covers all the rock.
The first is at 200X, the second is 400X.
Attachments
Similar threads
- Replies
- 1
- Views
- 129