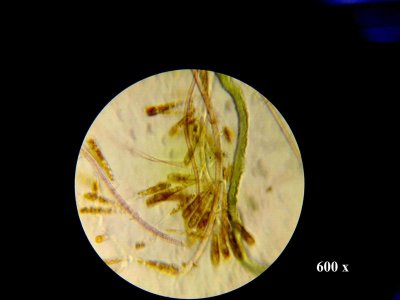
Before was on my 55g and I was harvesting calurpa and ulva with a touch of chaeto but chaeto never did very well compared to those two. This was in a custom built hang on back refugium.
I now use an ATS I bought from Turbo's Aquatics and it's fun seeing what I scrape off every couple weeks.
I feel the combination of what I've been doing for import and export which does include the ATS along with not freaking out and changing anything helped.
Post 2538
http://www.reefcentral.com/forums/showpost.php?p=24231040&postcount=2538
Yes, I've been carbon dosing for years and have dino's. None visually right now and probably wont see them again until I screw something up again. My parameters are pretty well posted around now to. With Nitrates under 1ppm and even under .5ppm. Phosphates around .03. Most recently under .03.
...
But I don't strive for clean or dirty and have no idea which I fall into. My parameters for nitrate and phosphates are low. But my sump is full of detritus I never touch. I don't use filter socks or other typical mechanical filtration. The flow in my display tank is setup now to keep detritus suspended until sucked down the overflow to my sump where it becomes food and home to many critters.
For exports:
"¢I don't run mechanical filters besides a skimmer which I use for aerating the water and removing bacteria that has consumed nitrates and phostphates.
"¢I've used vinegar for years and dosed ~100ml/day for 200g total water volume.
"¢I do ~1% automatic water changes daily exporting stuff.
"¢I harvest algae via an ATS.
For imports:
"¢I feed a lot. 4 times per day I have an automatic feeder dump some NLS Marine/AlgaeMax pellets. I hand feed 2-3x per day some meaty food maybe ~2-3 cubes worth. A sheet of nori per day. This brings in a lot of phosphates, trace elements, vitamins, minerals, etc. Plus increases urea dosing nitrates to my tank.
"¢~1% daily automatic water changes importing stuff
"¢I add a small amount of Mg to that water change water. I need to reduce that amount.
"¢I dose limewater which was via ATO and right now experimenting with limewater dosing separate from my ATO.
"¢Carbon dosing via vinegar ~100ml/day. But recently experimenting with a vodka/vinegar mix dosing ~36ml/day.
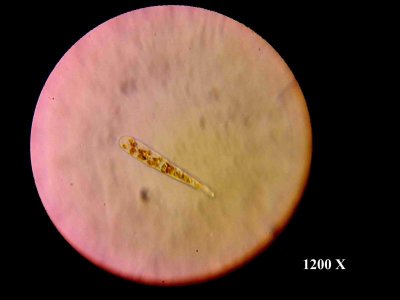
I didn't touch the dino's this time around and just watched them. Pictures of their peak and eventual decline earlier in this thread.
and to define a few things
Nitrates have always been below 5ppm. Since testing with other nitrate kits that can detect below 5ppm my nitrates are constantly below 1ppm.
Phosphates are consistently around .03ppm and recently lower. My last test was ~ 0.01
Vodka/vinegar mix are in a ratio mixed up to 690ml vinegar and 310ml of vodka based on TMZ's mix from the RedSea NOPOX thread exposing what's in it. No water added.
and my Mg supplement used to be Tech-m but switched a few years ago to a Mg Chloride/Sulfate I mix together myself now for cheaper. Using Randy's ratio for those using Kalk