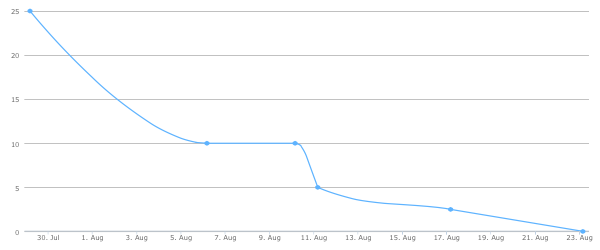
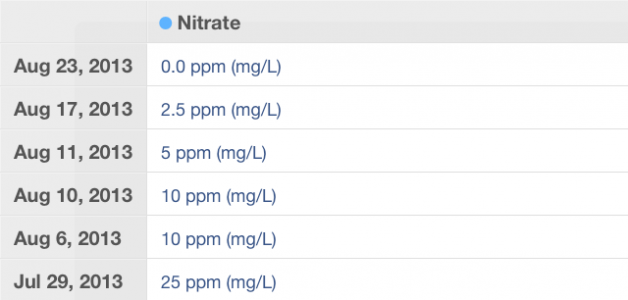
Ah.
There are lots of discussions on the reef chemistry forum back a few years about vodka and vinegar and some on any role anammox bacteria may or may not have. I had numerous discussions with Randy Farley about vodka vs vinegar. Both are well regarded. He often recommended either or both. They are essentially the same as ethanol oxidizes to acetic acid in water. I don't think anammox if they play any role in reef tanks prefer either one as a carbon source. Acetate is acetate whether it is from acetic acid, akak eth\anoic acid or ethanol.
As a practical matter; vodka can be dosed in bolus fashion; vinegar spikes pH downward and needs to be slow dosed over a long period of time during periods of photosynthesis. Vinegar is is more dilute requiring larger volumes of dosing which can make it easier to manage in some applications.
The article lays out a useful course for vinegar dosing.
The lead off paragraph focuses anammox bacteria which may be interpreted tio imply more significance to these bacteria than is warranted.
Cliff after reading some watewater treatment studies was interested in the anammox bacteria's role which is substantial in controlled wastewater treatment applications ( in vats with anoxic water, sludge sources,hydrazine additions etc). Hydrazine is a by product of their activity,btw ; it is used for jet fuel ; it is highly toxic. Often,unfortunately extrapolations form waste water treatment methods and studies to reef tanks miss the mark ,ime.
In wastewater treatment ,IIRC, acetic acid is used to encourage anammox bacteria in very controlled conditions unlike those in reef tanks . If acetic acid is favored in these treatments perhaps it's because the ethanol oxidation to acetic acid may somehow compete with the anammox bacteria in their vats. I really don't know and don't see the relevance in any case
ANammox bacteria also occur in anoxic regions of the deep ocean and in muddy anoxic areas. It is estimated that they account for 50% or so of the nitrogen removal in the oceans. They are obligate chemolitotrophs; they can not survive in the presence of oxygen as they are outdone by other bacteria like ammonia oxidizers and heterotrophic bacteria that thrive in areas where there is oxygen. Anammox bacteria are very slow growers by bacteria standards taking 10 days to two weeks to split. Other bacteria can do it in minutes to hours.
I am skeptical that the anammox play any signifcant role in a typcial reef tank. they might if there are enough anoxic areas. Even then ,I think sufate reducing bacteria would cmpetee with them. I hope they don't wax in a reef tak dosed with eithr vodka or vinegar since I don't want hydrazine in my tanks.
BTW,Anammox bacteria do not use nitrate as noted in the first paragraph of the article. They use ammonia and nitite . They are not part of the traditional nitrogen cycle as we know it.
Here is the anammox reaction:
NH<sub>4</sub><sup>+</sup> + NO<sub>2</sub><sup>-</sup> = N<sub>2</sub> + 2H<sub>2</sub>O
There are lots of discussions on the reef chemistry forum back a few years about vodka and vinegar and some on any role anammox bacteria may or may not have. I had numerous discussions with Randy Farley about vodka vs vinegar. Both are well regarded. He often recommended either or both. They are essentially the same as ethanol oxidizes to acetic acid in water. I don't think anammox if they play any role in reef tanks prefer either one as a carbon source. Acetate is acetate whether it is from acetic acid, akak eth\anoic acid or ethanol.
As a practical matter; vodka can be dosed in bolus fashion; vinegar spikes pH downward and needs to be slow dosed over a long period of time during periods of photosynthesis. Vinegar is is more dilute requiring larger volumes of dosing which can make it easier to manage in some applications.
The article lays out a useful course for vinegar dosing.
The lead off paragraph focuses anammox bacteria which may be interpreted tio imply more significance to these bacteria than is warranted.
Cliff after reading some watewater treatment studies was interested in the anammox bacteria's role which is substantial in controlled wastewater treatment applications ( in vats with anoxic water, sludge sources,hydrazine additions etc). Hydrazine is a by product of their activity,btw ; it is used for jet fuel ; it is highly toxic. Often,unfortunately extrapolations form waste water treatment methods and studies to reef tanks miss the mark ,ime.
In wastewater treatment ,IIRC, acetic acid is used to encourage anammox bacteria in very controlled conditions unlike those in reef tanks . If acetic acid is favored in these treatments perhaps it's because the ethanol oxidation to acetic acid may somehow compete with the anammox bacteria in their vats. I really don't know and don't see the relevance in any case
ANammox bacteria also occur in anoxic regions of the deep ocean and in muddy anoxic areas. It is estimated that they account for 50% or so of the nitrogen removal in the oceans. They are obligate chemolitotrophs; they can not survive in the presence of oxygen as they are outdone by other bacteria like ammonia oxidizers and heterotrophic bacteria that thrive in areas where there is oxygen. Anammox bacteria are very slow growers by bacteria standards taking 10 days to two weeks to split. Other bacteria can do it in minutes to hours.
I am skeptical that the anammox play any signifcant role in a typcial reef tank. they might if there are enough anoxic areas. Even then ,I think sufate reducing bacteria would cmpetee with them. I hope they don't wax in a reef tak dosed with eithr vodka or vinegar since I don't want hydrazine in my tanks.
BTW,Anammox bacteria do not use nitrate as noted in the first paragraph of the article. They use ammonia and nitite . They are not part of the traditional nitrogen cycle as we know it.
Here is the anammox reaction:
NH<sub>4</sub><sup>+</sup> + NO<sub>2</sub><sup>-</sup> = N<sub>2</sub> + 2H<sub>2</sub>O
Last edited: